
Which of the following carbocation is most stable?
(A)- ${{(C{{H}_{3}})}_{3}}C-CH_{2}^{+}$
(B)- ${{(C{{H}_{3}})}_{3}}{{C}^{+}}$
(C)- $C{{H}_{3}}C{{H}_{2}}CH_{2}^{+}$
(D)- $C{{H}_{3}}C{{H}^{+}}C{{H}_{2}}C{{H}_{3}}$
Answer
520.7k+ views
Hint: Carbocations are positive charged species formed as a result of heterolytic cleavage of bonds. Alkyl groups stabilize a carbocation by electron releasing effect. Stability of carbocations depends on the extent of dispersal of the positive charge and follows the order
\[{{3}^{o}}carbocation>{{2}^{o}}carbocation>{{1}^{o}}carbocation\]
Complete step by step solution:
Depending on the type of carbon bearing positive, carbocations can be primary (${{1}^{o}}$), secondary (${{2}^{o}}$) and tertiary (${{3}^{o}}$). ${{1}^{o}}$, ${{2}^{o}}$ and ${{3}^{o}}$ carbocation has positively charged carbon bonded to one, two and three other carbon groups, i.e.
\[\begin{matrix}
R-CH_{2}^{+} & R-C{{H}^{+}}-R & {{R}_{3}}{{C}^{+}} \\
{{1}^{o}} & {{2}^{o}} & {{3}^{o}} \\
\end{matrix}\]
Stability of carbocations can be explained by inductive effect (+I effect). An alkyl group is electron releasing in nature. So alkyl groups attached to a carbon bearing positive charge tend to stabilize that positive charge on the carbon. In doing so, the positive charge is dispersed on the alkyl groups. Now if a greater number of alkyl groups is attached to the positively charged carbon, there will be greater dispersal of positive charge on the alkyl groups and hence, more will be the stability of the carbocation.
Therefore, ${{3}^{o}}$ carbocation is most stable followed by ${{2}^{o}}$ and ${{1}^{o}}$ carbocations.
Let us look at the options given and classify them into ${{1}^{o}}$, ${{2}^{o}}$ and ${{3}^{o}}$ carbocation.
${{(C{{H}_{3}})}_{3}}C-CH_{2}^{+}$ and $C{{H}_{3}}C{{H}_{2}}CH_{2}^{+}$ have only one alkyl groups attached (${{1}^{o}}$) and thus have less +I effect.
$C{{H}_{3}}C{{H}^{+}}C{{H}_{2}}C{{H}_{3}}$ has two alkyl, i.e. one methyl and one ethyl group attached. Thus, being ${{2}^{o}}$carbocation it shows more +I effect than ${{1}^{o}}$ carbocation.
${{(C{{H}_{3}})}_{3}}{{C}^{+}}$ is ${{3}^{o}}$ carbocation as it has three methyl groups. +I effect will be more in case of ${{(C{{H}_{3}})}_{3}}{{C}^{+}}$ than ${{1}^{o}}$and ${{2}^{o}}$ carbocations.
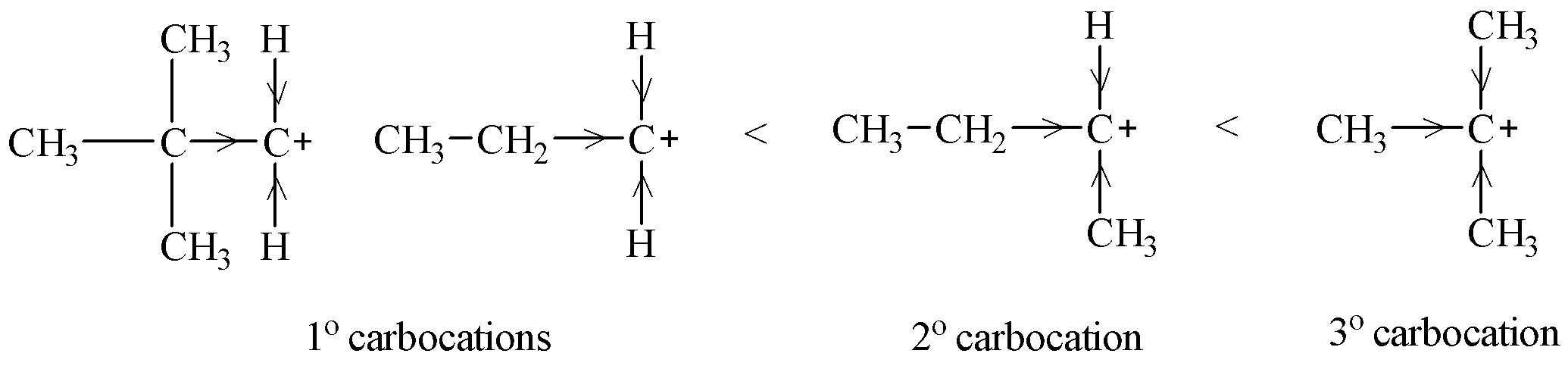
Therefore, ${{(C{{H}_{3}})}_{3}}{{C}^{+}}$ is the most stable carbocation.
Hence, the correct option is (B)
Note: Carefully classify the given carbocations into ${{1}^{o}}$, ${{2}^{o}}$ and ${{3}^{o}}$ to determine the stability. Greater the dispersal of positive charge more is the stability of the carbocation. Stability of carbocations that are attached to a benzene ring or double bond is explained by resonance and hyperconjugation effects.
\[{{3}^{o}}carbocation>{{2}^{o}}carbocation>{{1}^{o}}carbocation\]
Complete step by step solution:
Depending on the type of carbon bearing positive, carbocations can be primary (${{1}^{o}}$), secondary (${{2}^{o}}$) and tertiary (${{3}^{o}}$). ${{1}^{o}}$, ${{2}^{o}}$ and ${{3}^{o}}$ carbocation has positively charged carbon bonded to one, two and three other carbon groups, i.e.
\[\begin{matrix}
R-CH_{2}^{+} & R-C{{H}^{+}}-R & {{R}_{3}}{{C}^{+}} \\
{{1}^{o}} & {{2}^{o}} & {{3}^{o}} \\
\end{matrix}\]
Stability of carbocations can be explained by inductive effect (+I effect). An alkyl group is electron releasing in nature. So alkyl groups attached to a carbon bearing positive charge tend to stabilize that positive charge on the carbon. In doing so, the positive charge is dispersed on the alkyl groups. Now if a greater number of alkyl groups is attached to the positively charged carbon, there will be greater dispersal of positive charge on the alkyl groups and hence, more will be the stability of the carbocation.
Therefore, ${{3}^{o}}$ carbocation is most stable followed by ${{2}^{o}}$ and ${{1}^{o}}$ carbocations.
Let us look at the options given and classify them into ${{1}^{o}}$, ${{2}^{o}}$ and ${{3}^{o}}$ carbocation.
${{(C{{H}_{3}})}_{3}}C-CH_{2}^{+}$ and $C{{H}_{3}}C{{H}_{2}}CH_{2}^{+}$ have only one alkyl groups attached (${{1}^{o}}$) and thus have less +I effect.
$C{{H}_{3}}C{{H}^{+}}C{{H}_{2}}C{{H}_{3}}$ has two alkyl, i.e. one methyl and one ethyl group attached. Thus, being ${{2}^{o}}$carbocation it shows more +I effect than ${{1}^{o}}$ carbocation.
${{(C{{H}_{3}})}_{3}}{{C}^{+}}$ is ${{3}^{o}}$ carbocation as it has three methyl groups. +I effect will be more in case of ${{(C{{H}_{3}})}_{3}}{{C}^{+}}$ than ${{1}^{o}}$and ${{2}^{o}}$ carbocations.

Therefore, ${{(C{{H}_{3}})}_{3}}{{C}^{+}}$ is the most stable carbocation.
Hence, the correct option is (B)
Note: Carefully classify the given carbocations into ${{1}^{o}}$, ${{2}^{o}}$ and ${{3}^{o}}$ to determine the stability. Greater the dispersal of positive charge more is the stability of the carbocation. Stability of carbocations that are attached to a benzene ring or double bond is explained by resonance and hyperconjugation effects.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




