
Which of the following alkenes gives only acetic acid and on oxidation with potassium permanganate solution
A. Ethylene
B. $1-Butene$
C. Propene
D. $2-Butene$
Answer
360.9k+ views
Hint: Alkenes react with potassium permanganate to form vicinal diol, which is then further oxidised to carboxylic acid. $KMn{{O}_{4}}$ is a strong oxidising agent, able to oxidise carbon atoms if they are bonded to sufficiently weak bonds such as a pi bond, any polar bond for example carbon-oxygen bonds including aldehydes and alcohols.
Complete Step by Step Answer:
Alkenes are hydrocarbons containing carbon-carbon double bonds. As we know, a double bond consists of one sigma and one pi bond. A pi bond is a relatively weak bond as compared to a sigma bond. Hence potassium permanganate which is a strong oxidising agent can oxidise carbon atoms with a pi bond in alkenes.
In acidic medium potassium permanganate cleaves the double bond and forms carbonyl compounds. But the reaction does not stop here. The carbonyl compounds further oxidised to carboxylic acid.
Here we have four alkenes, upon oxidation with potassium permanganate producing carboxylic acid.
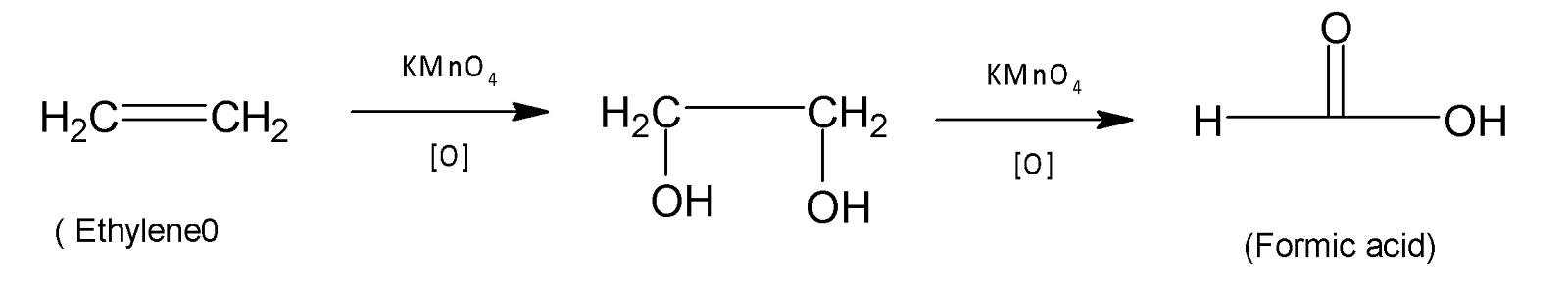
From (A) we have ethylene which undergoes oxidation to give acetic acid in the presence of potassium permanganate.
$1-Butene$ undergoes oxidation to give formic acid and propanoic acid in the presence of potassium permanganate.

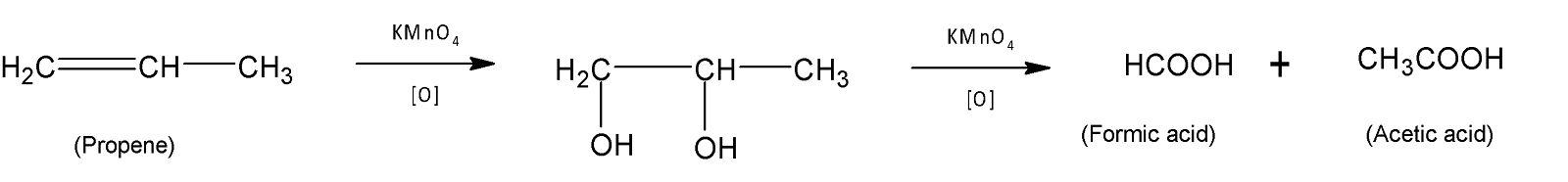
In option (C) propene gives formic acid and acetic acid upon oxidation in the presence of potassium permanganate.
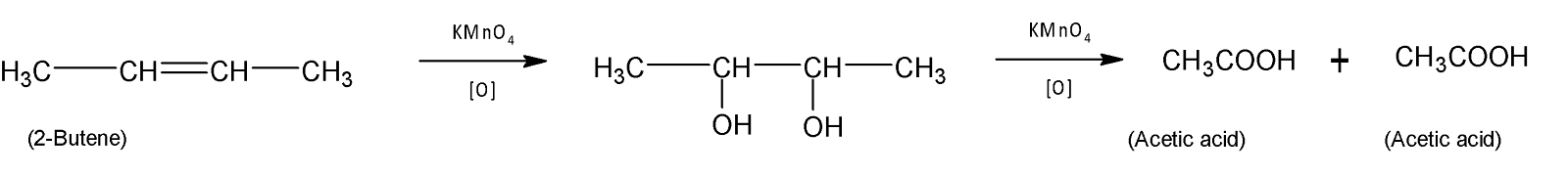
Finally $2-Butene$ produces only acetic acid with potassium permanganate.
Thus, option (D) is correct.
Note: Alkanes can not be oxidised by potassium permanganate as it consists of a more stable sigma bond and has no functional group for oxidation. But with tertiary hydrogen atoms, alkanes can be oxidised by acidic $KMn{{O}_{4}}$ to corresponding tertiary alcohol.
Complete Step by Step Answer:
Alkenes are hydrocarbons containing carbon-carbon double bonds. As we know, a double bond consists of one sigma and one pi bond. A pi bond is a relatively weak bond as compared to a sigma bond. Hence potassium permanganate which is a strong oxidising agent can oxidise carbon atoms with a pi bond in alkenes.
In acidic medium potassium permanganate cleaves the double bond and forms carbonyl compounds. But the reaction does not stop here. The carbonyl compounds further oxidised to carboxylic acid.
Here we have four alkenes, upon oxidation with potassium permanganate producing carboxylic acid.
From (A) we have ethylene which undergoes oxidation to give acetic acid in the presence of potassium permanganate.

$1-Butene$ undergoes oxidation to give formic acid and propanoic acid in the presence of potassium permanganate.

In option (C) propene gives formic acid and acetic acid upon oxidation in the presence of potassium permanganate.

Finally $2-Butene$ produces only acetic acid with potassium permanganate.

Thus, option (D) is correct.
Note: Alkanes can not be oxidised by potassium permanganate as it consists of a more stable sigma bond and has no functional group for oxidation. But with tertiary hydrogen atoms, alkanes can be oxidised by acidic $KMn{{O}_{4}}$ to corresponding tertiary alcohol.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Difference Between Plant Cell and Animal Cell




