
Which of the diene has the highest heat of hydrogenation?
A.
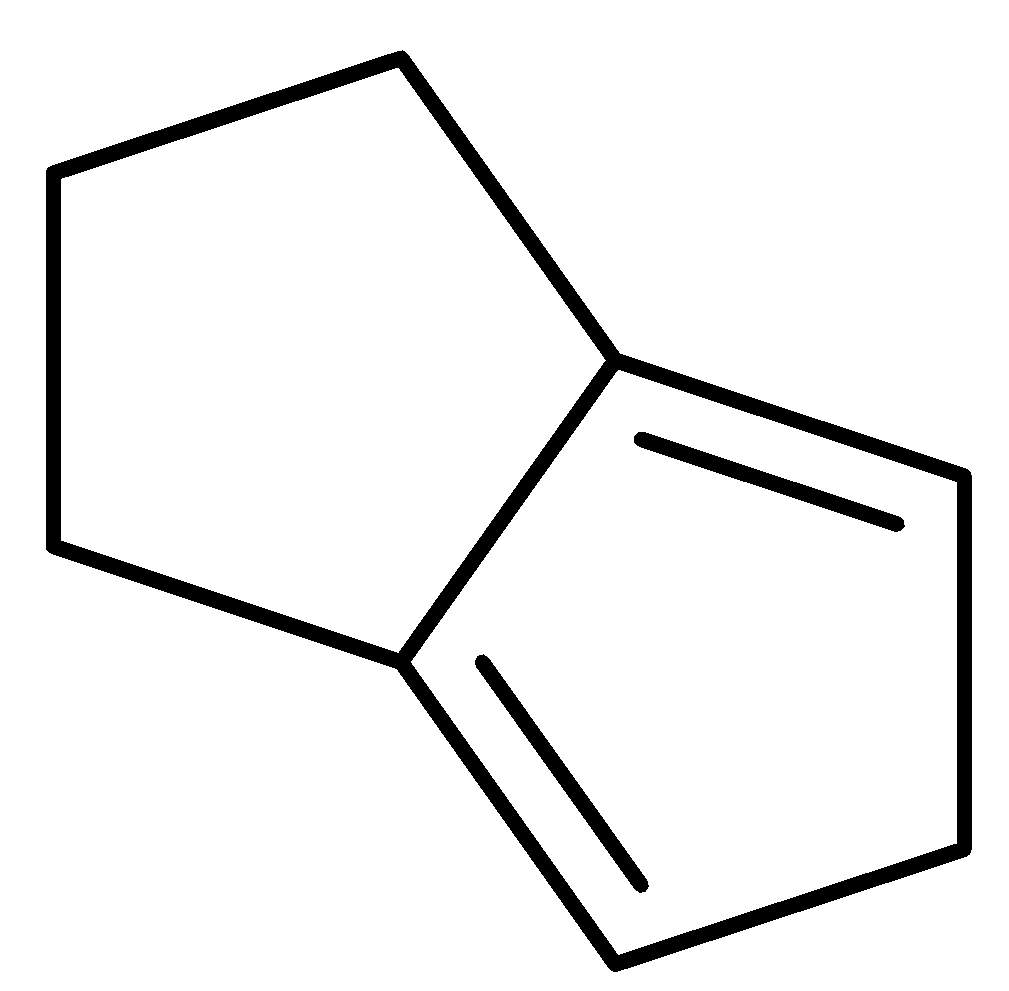
B.

C.
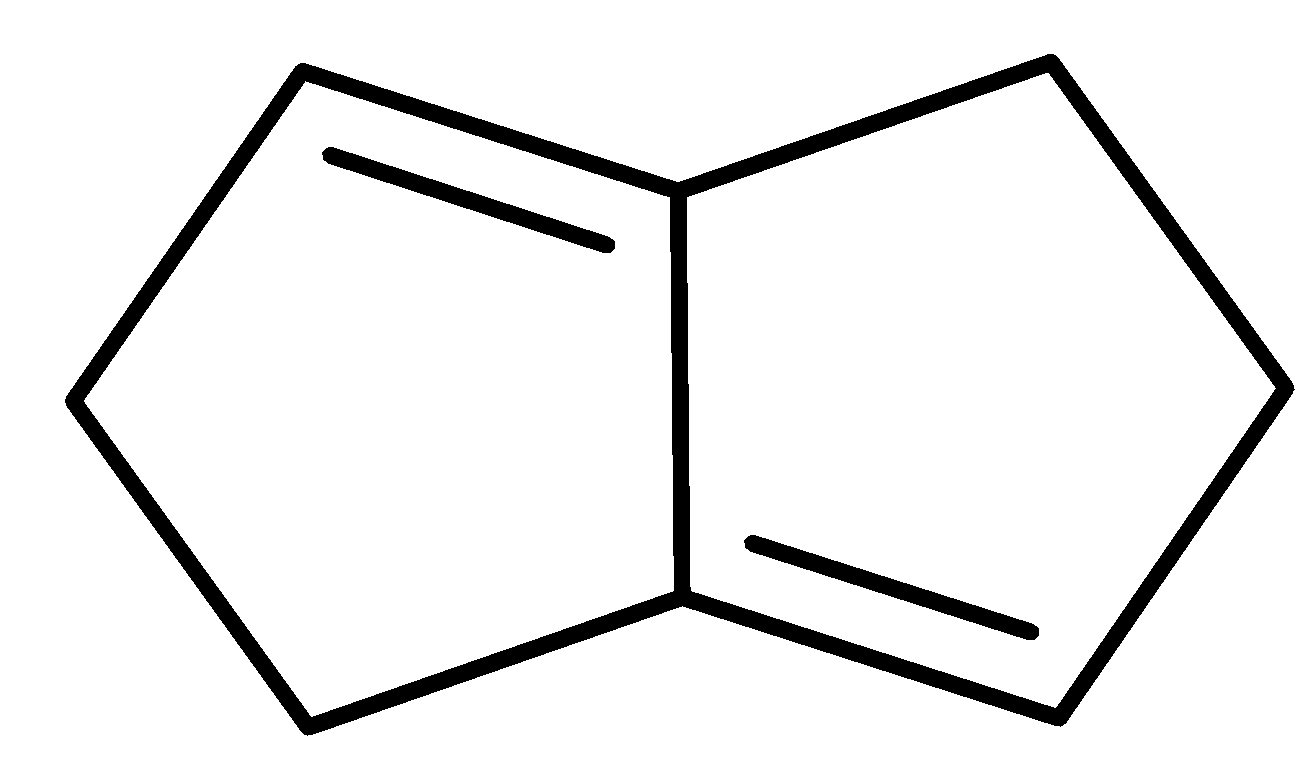
D.
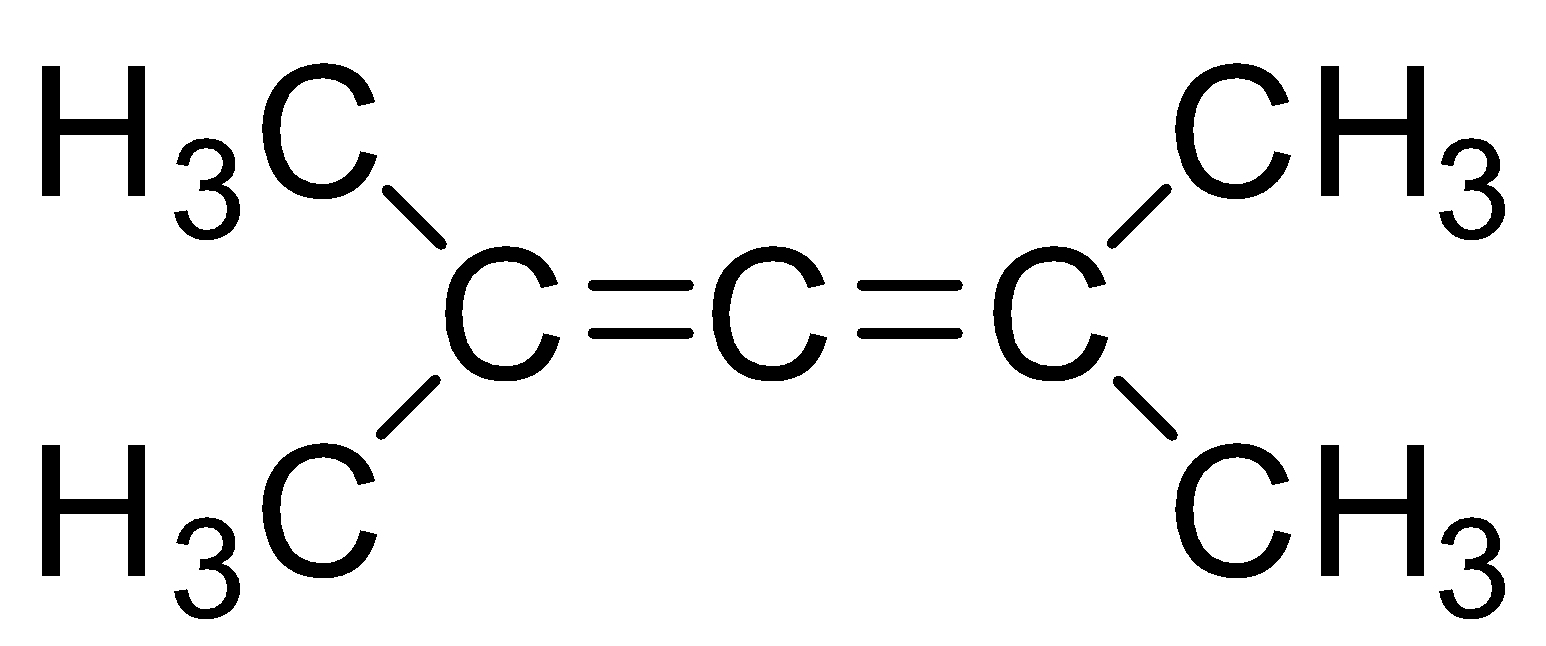



Answer
592.8k+ views
Hint: A diene is referred as a hydrocarbon which is unsaturated and having two double bonds between carbon atoms, there is one more term which one needs to know and that’s the heat of hydrogenation it is defined as a change in the enthalpy that a diene experienced when it is reacted with hydrogen which is in excess so that the diene is converted into the fully saturated compound.
Complete step by step answer:
Now, as we are familiar what diene and Heat of hydrogenation are let’s know what are the factors we need to consider in comparing the heat of hydrogenation of all the given above diene, the main factor is the stability of the alkenes or dienes, and this stability factor has an inverse relation with the heat of hydrogenation which means the more stable is the alkene the less will be the heat of hydrogenation.
Now, let’s identify the least stable diene as that diene will have the maximum heat of hydrogenation, when we learn about the stability of alkene or diene the conjugated one are the more stable (for a molecule to exhibit conjugation three adjacent p-orbitals should be present) then the non-conjugated or cumulated alkenes, and here the first three mention dienes are conjugated as there can be shifting of double bonds, which provides stability, whereas the fourth diene is cumulated and shifting of double bonds cannot take place hence is least stable diene and has a maximum heat of hydrogenation. The last diene does not shows conjugations can be easily shown with the help of diagram as follows:
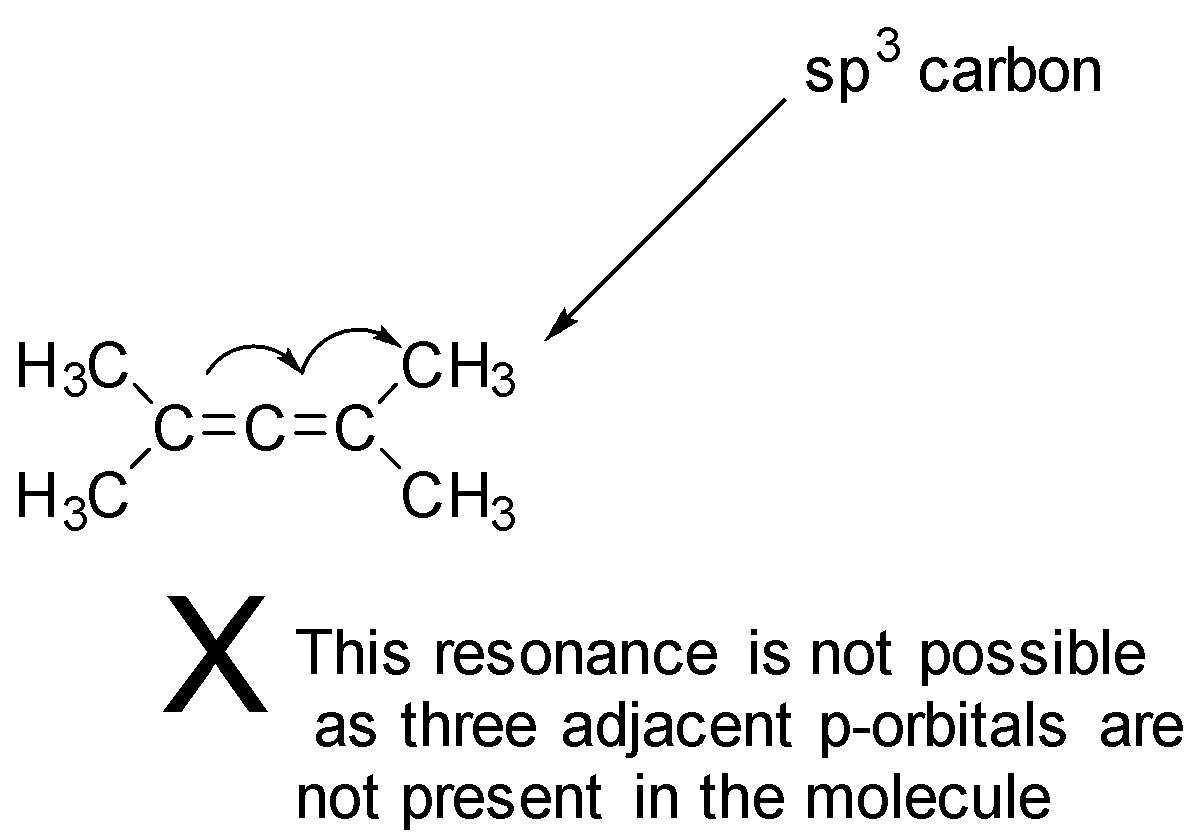
So, the correct answer is Option D.
Note:
For an alkene if we need to observe the Heat of hydrogenation it should be at atmospheric pressure and room temperature, the reactants should be in its natural states.
Complete step by step answer:
Now, as we are familiar what diene and Heat of hydrogenation are let’s know what are the factors we need to consider in comparing the heat of hydrogenation of all the given above diene, the main factor is the stability of the alkenes or dienes, and this stability factor has an inverse relation with the heat of hydrogenation which means the more stable is the alkene the less will be the heat of hydrogenation.
Now, let’s identify the least stable diene as that diene will have the maximum heat of hydrogenation, when we learn about the stability of alkene or diene the conjugated one are the more stable (for a molecule to exhibit conjugation three adjacent p-orbitals should be present) then the non-conjugated or cumulated alkenes, and here the first three mention dienes are conjugated as there can be shifting of double bonds, which provides stability, whereas the fourth diene is cumulated and shifting of double bonds cannot take place hence is least stable diene and has a maximum heat of hydrogenation. The last diene does not shows conjugations can be easily shown with the help of diagram as follows:

So, the correct answer is Option D.
Note:
For an alkene if we need to observe the Heat of hydrogenation it should be at atmospheric pressure and room temperature, the reactants should be in its natural states.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




