
Which L sugar on oxidation gives an optically active dibasic acid ( 2 - COOH groups)?
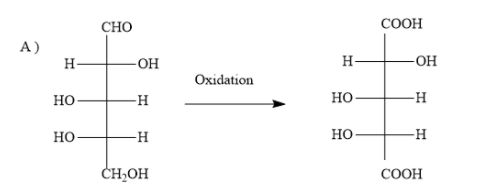
A)
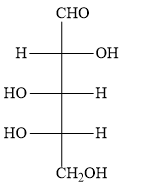
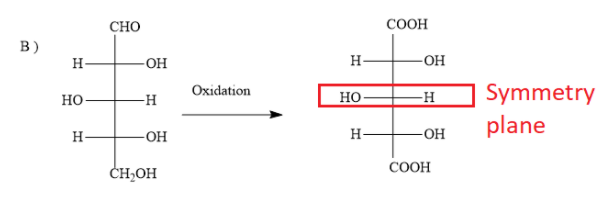
B)
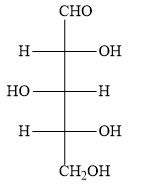
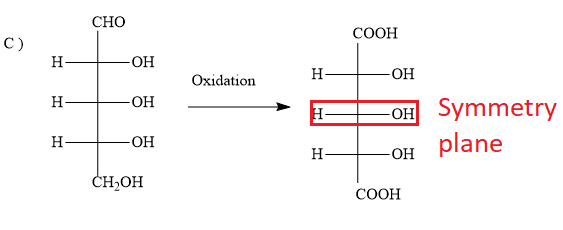
C)
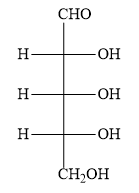
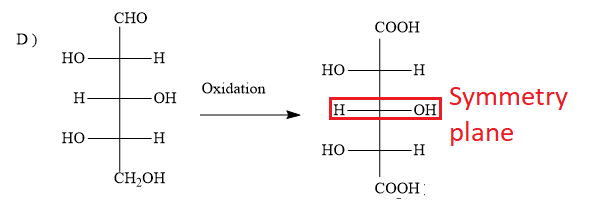
D)
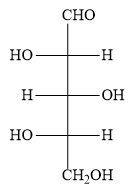




Answer
590.1k+ views
Hint: In the Fischer projection formula for D-sugar, the bottom most asymmetric carbon atom has hydroxyl group on the right. In the Fischer projection formula for L-sugar, the bottom most asymmetric carbon atom has hydroxyl group on the left. When a sugar molecule is oxidised, the product is a dibasic acid. If a plane of symmetry is present, the product is optically inactive.
Complete step by step answer:
The options B ) and C ) represent D- sugars as in the fischer projection formula, the bottom most asymmetric carbon atom has hydroxyl group on the right. But in the question, you have to find optically active dibasic acid from L-sugar. Hence, the options B ) and C ) are ruled out.
The options A ) and D ) represent L- sugars as in the fischer projection formula, the bottom most asymmetric carbon atom has hydroxyl group on the left.
When a sugar molecule is oxidised, the aldehyde group is converted to a carboxylic group. Similarly, the primary alcohol group is also oxidised to the carboxylic group. Thus, the oxidation of sugar gives a dicarboxylic acid. The product is dibasic acid with two carboxylic acid groups.
When the sugar in option D ) is oxidized, the product dicarboxylic acid contains a plane of symmetry (a mirror plane). Hence, the product is optically inactive. But in the question, you have to find optically active dibasic acid from L-sugar. Hence, the option D ) is ruled out.
When the sugar in option A ) is oxidized, the product dicarboxylic acid does not contain a plane of symmetry (a mirror plane). Hence, the product is optically active. In the question also, you have to find optically active dibasic acid from L-sugar.
Hence, the option A ) is the correct option.
Note: When a symmetry plane is present, the product will be optically inactive due to internal compensation. Such products are meso forms. When a symmetry plane is absent, the product is optically active and present as enantiomeric pairs.
Complete step by step answer:
The options B ) and C ) represent D- sugars as in the fischer projection formula, the bottom most asymmetric carbon atom has hydroxyl group on the right. But in the question, you have to find optically active dibasic acid from L-sugar. Hence, the options B ) and C ) are ruled out.
The options A ) and D ) represent L- sugars as in the fischer projection formula, the bottom most asymmetric carbon atom has hydroxyl group on the left.
When a sugar molecule is oxidised, the aldehyde group is converted to a carboxylic group. Similarly, the primary alcohol group is also oxidised to the carboxylic group. Thus, the oxidation of sugar gives a dicarboxylic acid. The product is dibasic acid with two carboxylic acid groups.
When the sugar in option D ) is oxidized, the product dicarboxylic acid contains a plane of symmetry (a mirror plane). Hence, the product is optically inactive. But in the question, you have to find optically active dibasic acid from L-sugar. Hence, the option D ) is ruled out.
When the sugar in option A ) is oxidized, the product dicarboxylic acid does not contain a plane of symmetry (a mirror plane). Hence, the product is optically active. In the question also, you have to find optically active dibasic acid from L-sugar.




Hence, the option A ) is the correct option.
Note: When a symmetry plane is present, the product will be optically inactive due to internal compensation. Such products are meso forms. When a symmetry plane is absent, the product is optically active and present as enantiomeric pairs.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE




