
Which is the incorrect match?
A.$F - C{H_2} - COOH > C{H_3} - COOH\,\,({K_a})$
B.
 (Stability)
(Stability)
C.
 (Stability)
(Stability)
D.
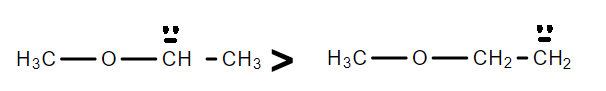 (Stability)
(Stability)


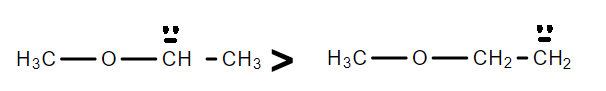
Answer
573.6k+ views
Hint: To answer this question, you should recall the concept of dipole moment and acidic character to compare the stability of different molecules. $ - {\text{I}}$ effect increases acidic strength and presence of resonance and hyperconjugation increases stability of a compound.
Complete Step by step solution:
Analyzing each option systematically:
A.In this question fluorine shows the\[ - {\text{I}}\] effect while \[ - C{H_3}\] shows \[{\text{ + I}}\] effect. As higher the electron-withdrawing effect stronger is the acidic character and higher is the ${K_a}$ value. Hence, this option is correct
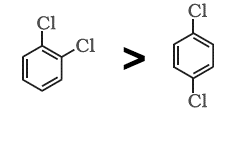
B.We know that the \[ - {\text{I}}\] effect of halides is stronger than the \[ + {\text{M}}\] effect. Hence the dipole moment of the first molecule larger than the second one. Thus this option is also correct
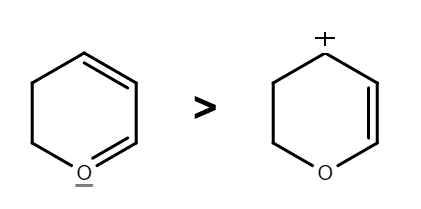
C.The positive charge in the first molecule is present on an oxygen atom which is more electronegative than carbon. While in the second molecule the positive charge is present on a carbon atom which is less electronegative than C atom. The structure in which the positive charge is present on the less electronegative atom is more stable. Hence, the second molecule is more stable. Thus, this option is incorrect
D.In the first molecule, the lone pair is in resonance with the oxygen atom, while in the second molecule there is no resonance effect involved with the lone pair.
Thus, the correct answer to this question is option C.
Note: When an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms, the corresponding negative or positive charge is relayed through the carbon chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect. Using the inductive effect, we can predict the acidity and basicity of compounds. It can be said as a generalisation the electron-withdrawing groups increase the acidity of a compound and electron-donating groups decrease the acidity of a compound. The charge on a given atom and the charge on a group bonded to the atom play a strong part when determining the stability of the resulting molecule as per the inductive effect. An example of this can be observed when a group displaying the \[ - {\text{I}}\]effect is bonded to a positively charged atom and the positive charge on the resulting molecule is amplified, reducing its stability. On the other hand, when a negatively charged atom is introduced to a group displaying an \[ - {\text{I}}\] effect, the charge disparity is somewhat quenched and the resulting molecule would be stable as per the inductive effect.
Complete Step by step solution:
Analyzing each option systematically:
A.In this question fluorine shows the\[ - {\text{I}}\] effect while \[ - C{H_3}\] shows \[{\text{ + I}}\] effect. As higher the electron-withdrawing effect stronger is the acidic character and higher is the ${K_a}$ value. Hence, this option is correct
B.We know that the \[ - {\text{I}}\] effect of halides is stronger than the \[ + {\text{M}}\] effect. Hence the dipole moment of the first molecule larger than the second one. Thus this option is also correct
C.The positive charge in the first molecule is present on an oxygen atom which is more electronegative than carbon. While in the second molecule the positive charge is present on a carbon atom which is less electronegative than C atom. The structure in which the positive charge is present on the less electronegative atom is more stable. Hence, the second molecule is more stable. Thus, this option is incorrect
D.In the first molecule, the lone pair is in resonance with the oxygen atom, while in the second molecule there is no resonance effect involved with the lone pair.
Thus, the correct answer to this question is option C.
Note: When an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms, the corresponding negative or positive charge is relayed through the carbon chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect. Using the inductive effect, we can predict the acidity and basicity of compounds. It can be said as a generalisation the electron-withdrawing groups increase the acidity of a compound and electron-donating groups decrease the acidity of a compound. The charge on a given atom and the charge on a group bonded to the atom play a strong part when determining the stability of the resulting molecule as per the inductive effect. An example of this can be observed when a group displaying the \[ - {\text{I}}\]effect is bonded to a positively charged atom and the positive charge on the resulting molecule is amplified, reducing its stability. On the other hand, when a negatively charged atom is introduced to a group displaying an \[ - {\text{I}}\] effect, the charge disparity is somewhat quenched and the resulting molecule would be stable as per the inductive effect.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




