
Which among the following free radicals is most stable?
A.
B.
C.

D.




Answer
569.7k+ views
Hint:
-Stability of radicals depends on a number of factors, like resonance and methyl substitution of the free radicalised carbon.
-With increase in resonating structures, the delocalisation of electron clouds increases, and the stability of the radical also increases.
Complete step by step answer:
A free radical is any molecular species which is capable of independent existence that consists of an unpaired electron in its atomic orbital. The presence of an unpaired electron results in some specific common properties which are shared by most of the radicals. Many radicals are known to be unstable and highly reactive species.
In order to understand the order of stability of these free radicals which are given in the option, we will consider some of the factors which determine the extent of stability of free radicals in general.
In case of alkanes, free radicals stabilised with increase in substitution of methyl groups, in other words, if we take an example of methyl, ethyl, and any other methyl substituted hydrocarbon like which is a secondary radical, then the secondary radical will be most stable, followed by the ethyl and then methyl will be least stable as it has least number of substitutions.
Now the second factor which determines stability is resonance, the more the resonance the more stable the free radical will be. This is because, we know that during resonance the electron cloud gets delocalised in the alternate double bonds and, so delocalisation of electron clouds makes the overall structure stable.
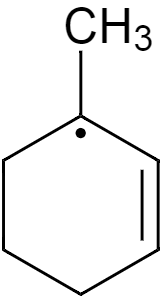
Now if we consider the question, the structure in first option has a double bond, and it is a tertiary allylic free radical, and a cyclic structure and the free radical is present on a carbon of the ring itself, which is adjacent to the double bond. So this free electron will participate in resonance and hence be highly stable.
Now if we consider the second option, the substituted carbon has the free electron, which is far from the double bond, so this electron will not participate in any resonance, hence not so stable.
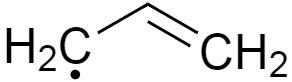
In case of third option, as we can see its methyl free radical and it is known to be most unstable of all because of its unsubstituted hydrogens, or more localised electron cloud.
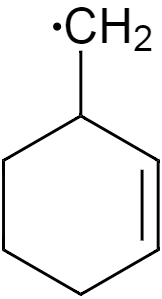
Lastly, the structure in option D, which is a secondary allylic free radical will also show resonance as the free radical is present adjacent to the double bond so it will get localised.
So the most appropriate option would be A.
Note:Tertiary allylic free radicals which have more hyperconjugation are more stable, as compared to secondary allylic free radicals because of lesser comparative hyperconjugative structures.
-The compounds which have more extent of resonance, will have more delocalised electron clouds, which results in more stability of the radical.
-Stability of radicals depends on a number of factors, like resonance and methyl substitution of the free radicalised carbon.
-With increase in resonating structures, the delocalisation of electron clouds increases, and the stability of the radical also increases.
Complete step by step answer:
A free radical is any molecular species which is capable of independent existence that consists of an unpaired electron in its atomic orbital. The presence of an unpaired electron results in some specific common properties which are shared by most of the radicals. Many radicals are known to be unstable and highly reactive species.
In order to understand the order of stability of these free radicals which are given in the option, we will consider some of the factors which determine the extent of stability of free radicals in general.
In case of alkanes, free radicals stabilised with increase in substitution of methyl groups, in other words, if we take an example of methyl, ethyl, and any other methyl substituted hydrocarbon like which is a secondary radical, then the secondary radical will be most stable, followed by the ethyl and then methyl will be least stable as it has least number of substitutions.
Now the second factor which determines stability is resonance, the more the resonance the more stable the free radical will be. This is because, we know that during resonance the electron cloud gets delocalised in the alternate double bonds and, so delocalisation of electron clouds makes the overall structure stable.
Now if we consider the question, the structure in first option has a double bond, and it is a tertiary allylic free radical, and a cyclic structure and the free radical is present on a carbon of the ring itself, which is adjacent to the double bond. So this free electron will participate in resonance and hence be highly stable.
Now if we consider the second option, the substituted carbon has the free electron, which is far from the double bond, so this electron will not participate in any resonance, hence not so stable.
In case of third option, as we can see its methyl free radical and it is known to be most unstable of all because of its unsubstituted hydrogens, or more localised electron cloud.
Lastly, the structure in option D, which is a secondary allylic free radical will also show resonance as the free radical is present adjacent to the double bond so it will get localised.
So the most appropriate option would be A.
Note:Tertiary allylic free radicals which have more hyperconjugation are more stable, as compared to secondary allylic free radicals because of lesser comparative hyperconjugative structures.
-The compounds which have more extent of resonance, will have more delocalised electron clouds, which results in more stability of the radical.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




