
What is the Saytzeff rule?
Answer
520.7k+ views
Hint:This rule is commonly used in organic chemistry. It helps in the prediction of the higher yield alkene when a particular compound undergoes elimination reaction. It is also known by the name Saytzeff - Hofmann rule.
Complete step by step solution:
Saytzeff’s rule, also known as Zaitsev’s rule is a rule in organic chemistry which is used to find out the favoured alkene product in an elimination reaction. In a variety of elimination reactions, a general trend was observed in the resulting alkenes. Based on this general trend of the alkene products, Saytzeff’s rule was coined.
We can write the actual statement of the rule as- "The alkene formed in the greatest amount is the one that corresponds to removal of the hydrogen from the alpha-carbon having the fewest hydrogen substituents."
As we can see, the rule suggests that among the two or more alkene products formed, the more substituted product will be the major product.
We can understand the rule better from the following example:
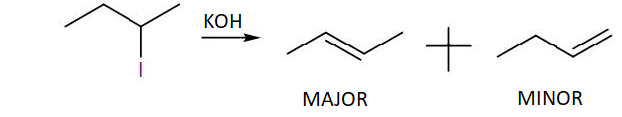
When 2-iodobutane which is the reactant in the above reaction, is treated with alcoholic potassium hydroxide, we obtain two products in which both are alkenes. The major product is 2-butene and the minor product is 1-butene.
As we can see in the structure of the major product, 2-butene, the double bond in the position of the alpha carbon has a higher number of substituent than the minor product, 1-butene, which has the double bond at the terminal carbon.
However, the rule makes no predictions about the stereochemistry of the product. It only explains the preference of breaking of the chemical bonding of the newly formed alkenes.
Additional information: The products formed depend on many other factors like stereochemistry and steric effect.
Note: It is important to remember here that there are exceptions to this rule too as we are predicting the product on a theoretical basis.
However, it is important to consider the alpha carbon position while predicting the product. Alpha carbon is the first carbon atom attached to the functional group.
Complete step by step solution:
Saytzeff’s rule, also known as Zaitsev’s rule is a rule in organic chemistry which is used to find out the favoured alkene product in an elimination reaction. In a variety of elimination reactions, a general trend was observed in the resulting alkenes. Based on this general trend of the alkene products, Saytzeff’s rule was coined.
We can write the actual statement of the rule as- "The alkene formed in the greatest amount is the one that corresponds to removal of the hydrogen from the alpha-carbon having the fewest hydrogen substituents."
As we can see, the rule suggests that among the two or more alkene products formed, the more substituted product will be the major product.
We can understand the rule better from the following example:

When 2-iodobutane which is the reactant in the above reaction, is treated with alcoholic potassium hydroxide, we obtain two products in which both are alkenes. The major product is 2-butene and the minor product is 1-butene.
As we can see in the structure of the major product, 2-butene, the double bond in the position of the alpha carbon has a higher number of substituent than the minor product, 1-butene, which has the double bond at the terminal carbon.
However, the rule makes no predictions about the stereochemistry of the product. It only explains the preference of breaking of the chemical bonding of the newly formed alkenes.
Additional information: The products formed depend on many other factors like stereochemistry and steric effect.
Note: It is important to remember here that there are exceptions to this rule too as we are predicting the product on a theoretical basis.
However, it is important to consider the alpha carbon position while predicting the product. Alpha carbon is the first carbon atom attached to the functional group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




