
What is the bond order of \[N{{O}^{+}}\] ?
Answer
594.9k+ views
Hint: \[N{{O}^{+}}\] has one electron less than that of NO. Formula to find bond order is as follows.
\[\text{Bond order = }\dfrac{1}{2}\text{(no}\text{. of }{{\text{e}}^{-}}\text{ in bonding orbitals - no}\text{. of }{{\text{e}}^{-}}\text{ in antibonding orbitals)}\]
Complete answer:
- Bond order simply represents the number of bonds between two atoms. It was introduced by Linus Pauling.
- Higher the bond order of a bond, higher the stability of the bond. Hence can say that a single bond is weaker than a double bond and a double bond is weaker than a triple bond.
- Here, \[N{{O}^{+}}\] has bond order equal to 3 that simply means that there is a triple bond present in between oxygen and nitrogen.
- In order to find the bond order, we need to have information about the molecular orbital diagram of the molecule. Molecular orbital diagram shows which electrons are present in bonding orbitals and which are present in antibonding orbitals.
- In some cases, we get bond order as a fractional number also.
Let’s see how much electrons are present in antibonding orbitals and bonding orbitals of \[N{{O}^{+}}\] in order to determine the bond order by MO diagram.
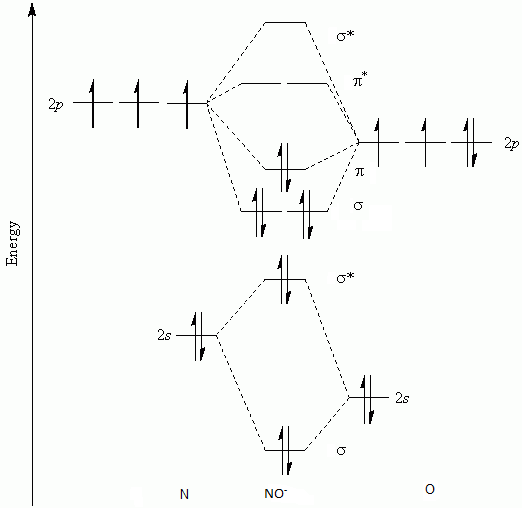
We can see that there are a total 2+2+2+2=8 electrons in the bonding orbitals.
There are only 2 electrons in the antibonding orbitals as we have removed one electron from \[{{\pi }^{*}}\] antibonding orbital. So, calculating the bond order by following formula, we get
\[\text{Bond order = }\dfrac{1}{2}\text{(no}\text{. of }{{\text{e}}^{-}}\text{ in bonding orbitals - no}\text{. of }{{\text{e}}^{-}}\text{ in antibonding orbitals)}\]
\[\text{Bond order = }\dfrac{1}{2}\text{(8 - 2)}\]
\[\text{Bond order = }\dfrac{1}{2}\text{(6)}\]
\[\text{Bond order = 3}\]
So, \[N{{O}^{+}}\] has bond order 3.
Note: Keep noted that antibonding orbitals have higher energy than bonding orbitals and they are filled after the bonding orbitals are filled. Do not forget to remove or add electrons in order to match the electronic charge on the molecule.
\[\text{Bond order = }\dfrac{1}{2}\text{(no}\text{. of }{{\text{e}}^{-}}\text{ in bonding orbitals - no}\text{. of }{{\text{e}}^{-}}\text{ in antibonding orbitals)}\]
Complete answer:
- Bond order simply represents the number of bonds between two atoms. It was introduced by Linus Pauling.
- Higher the bond order of a bond, higher the stability of the bond. Hence can say that a single bond is weaker than a double bond and a double bond is weaker than a triple bond.
- Here, \[N{{O}^{+}}\] has bond order equal to 3 that simply means that there is a triple bond present in between oxygen and nitrogen.
- In order to find the bond order, we need to have information about the molecular orbital diagram of the molecule. Molecular orbital diagram shows which electrons are present in bonding orbitals and which are present in antibonding orbitals.
- In some cases, we get bond order as a fractional number also.
Let’s see how much electrons are present in antibonding orbitals and bonding orbitals of \[N{{O}^{+}}\] in order to determine the bond order by MO diagram.

We can see that there are a total 2+2+2+2=8 electrons in the bonding orbitals.
There are only 2 electrons in the antibonding orbitals as we have removed one electron from \[{{\pi }^{*}}\] antibonding orbital. So, calculating the bond order by following formula, we get
\[\text{Bond order = }\dfrac{1}{2}\text{(no}\text{. of }{{\text{e}}^{-}}\text{ in bonding orbitals - no}\text{. of }{{\text{e}}^{-}}\text{ in antibonding orbitals)}\]
\[\text{Bond order = }\dfrac{1}{2}\text{(8 - 2)}\]
\[\text{Bond order = }\dfrac{1}{2}\text{(6)}\]
\[\text{Bond order = 3}\]
So, \[N{{O}^{+}}\] has bond order 3.
Note: Keep noted that antibonding orbitals have higher energy than bonding orbitals and they are filled after the bonding orbitals are filled. Do not forget to remove or add electrons in order to match the electronic charge on the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




