
What is the action of chlorine on ${{C}}{{{S}}_{{2}}}$
Answer
565.5k+ views
Hint: We know that ${{C}}{{{S}}_{{2}}}$ is a volatile colourless liquid. It is called carbon disulphide. The structure of ${{C}}{{{S}}_{{2}}}$ is
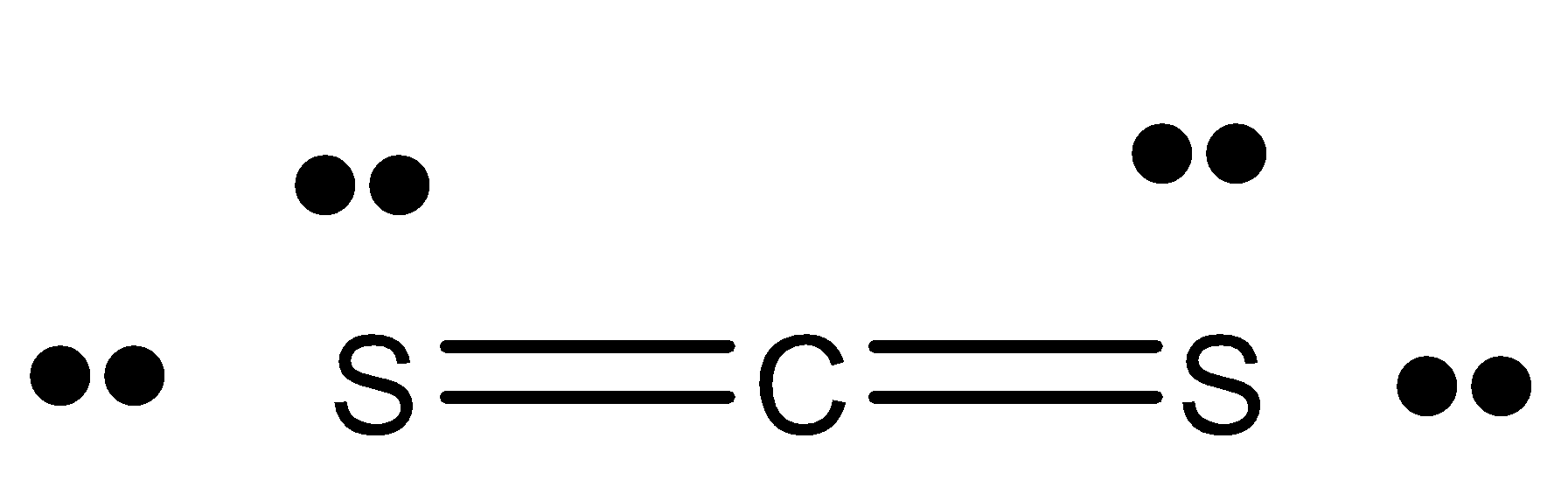 .
.
(Carbon has 4 valence electrons, Sulphur has 6 valence electrons. In ${{C}}{{{S}}_{{2}}}$ , there are 2 Sulphur atoms, so 12 valence electrons. Thus, the total electrons is 16). Now, there is a double bond in ${{C}}{{{S}}_{{2}}}$ and 2 lone pairs on each Sulphur atom. It is used as a chemical non-polar solvent. It belongs to a class of inorganic compounds known as non-metallic sulphides. It is used as a building block in organic chemistry.
Complete step by step answer:
We know that chlorine is a halogen. ${{C}}{{{l}}_{{2}}}$ molecules when reacts with sodium atom produces sodium chloride
When sulphur and carbon are combined at high temperatures, carbon disulphide or carbon disulfide is formed.
\[C + 2S \to C{S_2}\]
Carbon disulphide can be produced from volcanic eruptions also.
We know that chlorine molecule is used as a chlorinating agent, to add chlorine to molecules.
When chlorine is treated with carbon disulphide, we get carbon tetrachloride.
${{C}}{{{S}}_{{2}}}{{ + 3C}}{{{l}}_{{2}}} \to {{CC}}{{{l}}_{{4}}}{{ + }}{{{S}}_{{2}}}{{C}}{{{l}}_{{2}}}$
We could see that chlorine replaces the sulphur in carbon disulphide.
Now, on adding ${{C}}{{{l}}_{{2}}}$ , it gets chlorinated and all the lone pairs on chlorine are satisfied by a chlorine atom, forming carbon tetrachloride.
Thus, the action of chlorine on ${{C}}{{{S}}_{{2}}}$ is the formation of carbon tetrachloride ${{CC}}{{{l}}_{{4}}}$
Note:
We know the uses of the product Carbon Tetrachloride of this reaction. Chlorine is a better leaving group. It is used as a chlorine source in many reactions. Freons which are used in AC and refrigerators are made using ${{CC}}{{{l}}_{{4}}}$ . It is used in fire extinguishers as it has a high melting point and low boiling point. Prolonged exposure can cause so much damage to humans.

(Carbon has 4 valence electrons, Sulphur has 6 valence electrons. In ${{C}}{{{S}}_{{2}}}$ , there are 2 Sulphur atoms, so 12 valence electrons. Thus, the total electrons is 16). Now, there is a double bond in ${{C}}{{{S}}_{{2}}}$ and 2 lone pairs on each Sulphur atom. It is used as a chemical non-polar solvent. It belongs to a class of inorganic compounds known as non-metallic sulphides. It is used as a building block in organic chemistry.
Complete step by step answer:
We know that chlorine is a halogen. ${{C}}{{{l}}_{{2}}}$ molecules when reacts with sodium atom produces sodium chloride
When sulphur and carbon are combined at high temperatures, carbon disulphide or carbon disulfide is formed.
\[C + 2S \to C{S_2}\]
Carbon disulphide can be produced from volcanic eruptions also.
We know that chlorine molecule is used as a chlorinating agent, to add chlorine to molecules.
When chlorine is treated with carbon disulphide, we get carbon tetrachloride.
${{C}}{{{S}}_{{2}}}{{ + 3C}}{{{l}}_{{2}}} \to {{CC}}{{{l}}_{{4}}}{{ + }}{{{S}}_{{2}}}{{C}}{{{l}}_{{2}}}$
We could see that chlorine replaces the sulphur in carbon disulphide.
Now, on adding ${{C}}{{{l}}_{{2}}}$ , it gets chlorinated and all the lone pairs on chlorine are satisfied by a chlorine atom, forming carbon tetrachloride.
Thus, the action of chlorine on ${{C}}{{{S}}_{{2}}}$ is the formation of carbon tetrachloride ${{CC}}{{{l}}_{{4}}}$
Note:
We know the uses of the product Carbon Tetrachloride of this reaction. Chlorine is a better leaving group. It is used as a chlorine source in many reactions. Freons which are used in AC and refrigerators are made using ${{CC}}{{{l}}_{{4}}}$ . It is used in fire extinguishers as it has a high melting point and low boiling point. Prolonged exposure can cause so much damage to humans.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




