
What is glycosidic linkage?
Answer
590.7k+ views
Hint: To solve this question we must know about the types of bonds in various chemical compounds. The different compounds are formed due to bonds formed between the corresponding atoms of any molecules. Ionic bond, covalent bond, hydrogen bond and van der wall’s interaction are the types of bond present between the various chemical compounds.
Complete step by step solution:
Glycosidic linkage occurs between the molecules of two monosaccharides through an oxygen atom and is accompanied by the loss of water molecule. Glycosidic linkage is a type of covalent bond.
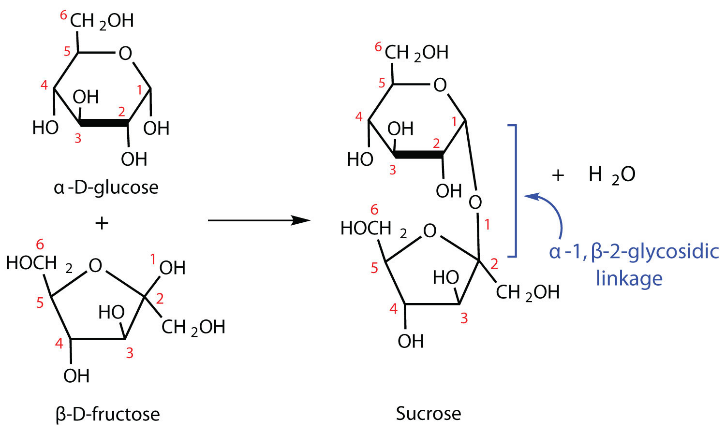
Additional Information:
Similarly, an ionic bond is formed between a positively charged and a negatively charged ions. An atom donates an electron from its outer shell making it positively charged and another atom accepts that electron making it negatively charged and an ionic bond is formed between the two ions. Example of an ionic bond is NaCl.
A covalent bond is formed due to the sharing of electrons between two atoms. Example\[{\text{C}}{{\text{H}}_{\text{4}}}\] .
Hydrogen bond is a polar covalent bond between a positively charged hydrogen atom and an electronegative atom (for example Cl, F etc.) in the same or different molecules.
Note: Monosaccharides are the simplest form of sugar that cannot be hydrolysed to form a simple chemical compound. Disaccharides are formed when two monosaccharides are joined to form a single molecule by a glycosidic linkage.
Complete step by step solution:
Glycosidic linkage occurs between the molecules of two monosaccharides through an oxygen atom and is accompanied by the loss of water molecule. Glycosidic linkage is a type of covalent bond.

Additional Information:
Similarly, an ionic bond is formed between a positively charged and a negatively charged ions. An atom donates an electron from its outer shell making it positively charged and another atom accepts that electron making it negatively charged and an ionic bond is formed between the two ions. Example of an ionic bond is NaCl.
A covalent bond is formed due to the sharing of electrons between two atoms. Example\[{\text{C}}{{\text{H}}_{\text{4}}}\] .
Hydrogen bond is a polar covalent bond between a positively charged hydrogen atom and an electronegative atom (for example Cl, F etc.) in the same or different molecules.
Note: Monosaccharides are the simplest form of sugar that cannot be hydrolysed to form a simple chemical compound. Disaccharides are formed when two monosaccharides are joined to form a single molecule by a glycosidic linkage.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




