
What are synthetic detergents?
Answer
598.5k+ views
Hint:
Water is said to be hard when it does not readily form lather with soap and is said to be soft, if it readily forms lather with soap. Synthetic detergents are chemical substances which are used in place of soap in the domestic and laundry work.
Step-by-step explanation:
Step 1:
Soap is a sodium salt of stearic acid having a formula \[{C_{17}}{H_{35}}COONa\], can be represented as \[NaSt\]. If the water is hard, then the calcium and the magnesium ions of the hard water react with the anion of the soap to form a slimy precipitate known as soap-curd or scum.
\[2NaSt + Ca{(HC{O_3})_2}\xrightarrow{{}}CaS{t_2} \downarrow + 2NaHC{O_3}\]
(soap) (scum)
Step 2:
Till the time the soap curd is present no soap lather will be formed and cleaning of cloth or body will not be possible.
Step 3:
This difficulty is resolved to a great extent by using synthetic detergents. For domestic and laundry purposes nowadays, synthetic detergents like Surf, Ariel are used. These are made by sulfonating some higher alkenes with sulphuric acid and then converting them into their sodium salts by caustic soda.
Step 4:
When the detergents are dissolved in water, the detergent molecules aggregate together to form micelles which have a hydrophobic hydrocarbon tail and a hydrophilic ionic head. Their tail sticks inward and the head outwards.
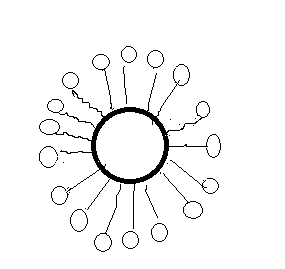
Step 5:
In cleansing, the hydrocarbon tail attaches itself to oily dirt. When the water is stirred, the oily dirt dissociates into fragments and other tails continuously stick to the oil. The solution now contains small globules of oil surrounded by detergent molecules. The ionic head does not allow the oil to aggregate and helps in removing the dirt.
Hence, the synthetic detergents are chemical substances which are used in place of soap in domestic and laundry work. They are more soluble in water and are not affected by the hardness of water as their calcium and magnesium salts are soluble in water.
Note: Detergents are synthetically prepared to overcome the problems faced with soap.
Water is said to be hard when it does not readily form lather with soap and is said to be soft, if it readily forms lather with soap. Synthetic detergents are chemical substances which are used in place of soap in the domestic and laundry work.
Step-by-step explanation:
Step 1:
Soap is a sodium salt of stearic acid having a formula \[{C_{17}}{H_{35}}COONa\], can be represented as \[NaSt\]. If the water is hard, then the calcium and the magnesium ions of the hard water react with the anion of the soap to form a slimy precipitate known as soap-curd or scum.
\[2NaSt + Ca{(HC{O_3})_2}\xrightarrow{{}}CaS{t_2} \downarrow + 2NaHC{O_3}\]
(soap) (scum)
Step 2:
Till the time the soap curd is present no soap lather will be formed and cleaning of cloth or body will not be possible.
Step 3:
This difficulty is resolved to a great extent by using synthetic detergents. For domestic and laundry purposes nowadays, synthetic detergents like Surf, Ariel are used. These are made by sulfonating some higher alkenes with sulphuric acid and then converting them into their sodium salts by caustic soda.
Step 4:
When the detergents are dissolved in water, the detergent molecules aggregate together to form micelles which have a hydrophobic hydrocarbon tail and a hydrophilic ionic head. Their tail sticks inward and the head outwards.

Step 5:
In cleansing, the hydrocarbon tail attaches itself to oily dirt. When the water is stirred, the oily dirt dissociates into fragments and other tails continuously stick to the oil. The solution now contains small globules of oil surrounded by detergent molecules. The ionic head does not allow the oil to aggregate and helps in removing the dirt.
Hence, the synthetic detergents are chemical substances which are used in place of soap in domestic and laundry work. They are more soluble in water and are not affected by the hardness of water as their calcium and magnesium salts are soluble in water.
Note: Detergents are synthetically prepared to overcome the problems faced with soap.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




