
What are isomers?
Answer
568.2k+ views
Hint: Isomers is a term used to group two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties.
Complete step by step answer:
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulas that have the same number of atoms of each element but distinct arrangements of atoms in space. Isomerism is the existence or possibility of isomers.
Isomers do not necessarily share similar chemical or physical properties.
The word ‘isomer’ obtained from back formation of “isomeric” which was borrowed through Greek ‘isomers’ where isos means ‘equal’ and meros means ‘part’.
There are two types of isomers
(1) Structural isomers
(2) Stereoisomers
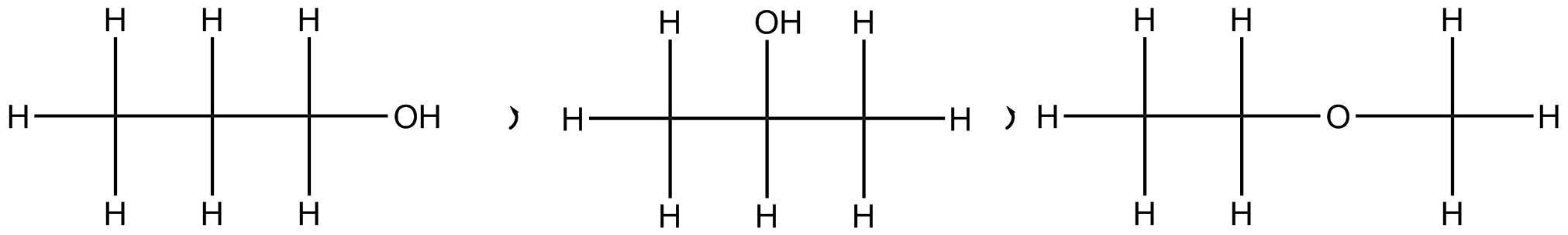
The compounds having the same structural formula but different properties are called structural isomers.
One more example of ${C_3}{H_4}$
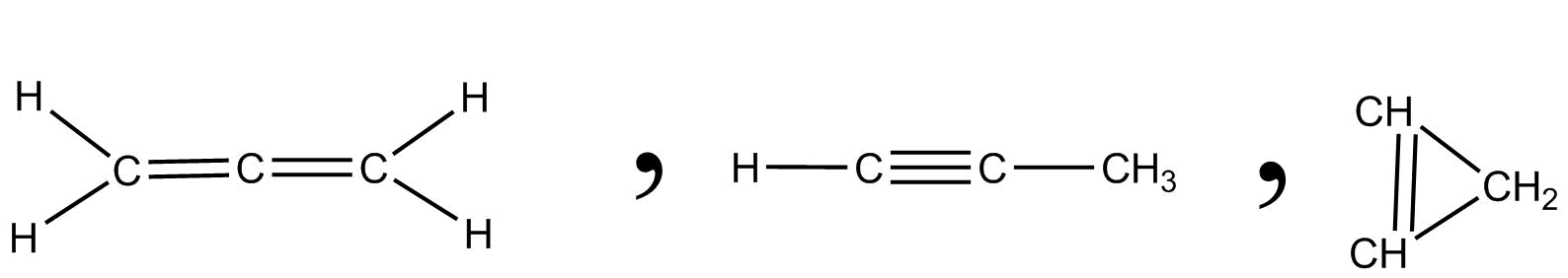
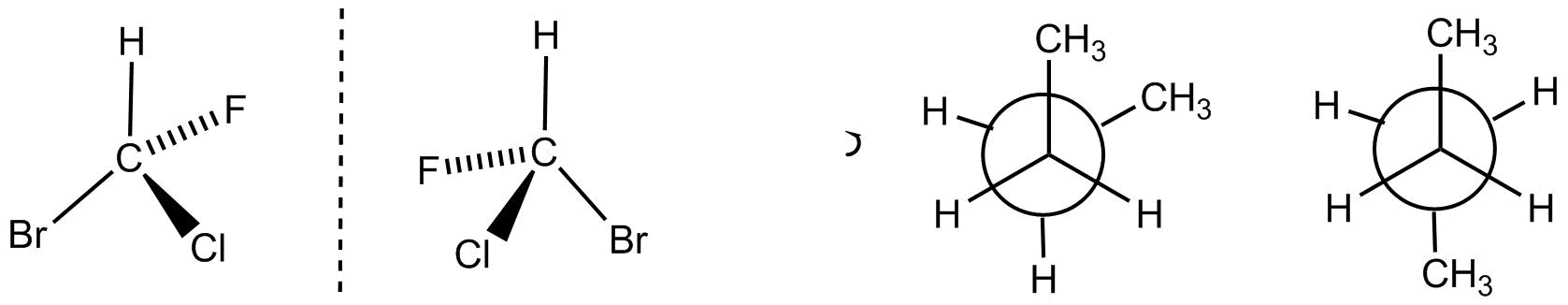
Stereoisomers have the same atoms or isotopes connected by bonds of the same shape type, but differ in their shape. The relative positions of these atoms in space, apart from rotations and translation.
Additional Information: The same isomers can also be in different excited states, that differ by quantum states of their electrons. For example, the oxygen molecule can be in triplet state or one of the two singlet states. These are not considered different isomers, since such molecules usually decay spontaneously to their lowest excitation states in a relatively short time scale.
Likewise, polyatomic ions and molecules that differ only by addition or removal of electrons, like oxygen or the peroxide ion.
Moreover, there is a type of isomers i.e. Tautomers. Tautomers are structural isomers which are readily interconvertible so that two species can coexist in equilibrium. Important examples are keto-enol tautomerism and neutral and zwitterionic form of amino acid.
Note: Isomers are very important in air pollution chemistry because even slightly different substances or structures can evoke dramatical differences in chemical and physical properties.
Complete step by step answer:
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulas that have the same number of atoms of each element but distinct arrangements of atoms in space. Isomerism is the existence or possibility of isomers.
Isomers do not necessarily share similar chemical or physical properties.
The word ‘isomer’ obtained from back formation of “isomeric” which was borrowed through Greek ‘isomers’ where isos means ‘equal’ and meros means ‘part’.
There are two types of isomers
(1) Structural isomers
(2) Stereoisomers
The compounds having the same structural formula but different properties are called structural isomers.

One more example of ${C_3}{H_4}$
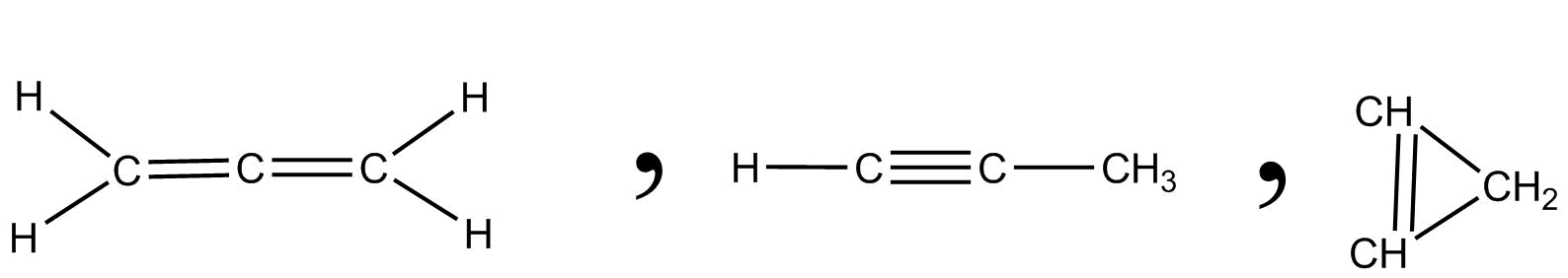
Stereoisomers have the same atoms or isotopes connected by bonds of the same shape type, but differ in their shape. The relative positions of these atoms in space, apart from rotations and translation.

Additional Information: The same isomers can also be in different excited states, that differ by quantum states of their electrons. For example, the oxygen molecule can be in triplet state or one of the two singlet states. These are not considered different isomers, since such molecules usually decay spontaneously to their lowest excitation states in a relatively short time scale.
Likewise, polyatomic ions and molecules that differ only by addition or removal of electrons, like oxygen or the peroxide ion.
Moreover, there is a type of isomers i.e. Tautomers. Tautomers are structural isomers which are readily interconvertible so that two species can coexist in equilibrium. Important examples are keto-enol tautomerism and neutral and zwitterionic form of amino acid.
Note: Isomers are very important in air pollution chemistry because even slightly different substances or structures can evoke dramatical differences in chemical and physical properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




