
How many water molecules are produced in the complete neutralization of one mole phosphoric acid with potassium hydroxide?
Answer
582.9k+ views
Hint: In a neutralization reaction, the acid and the base quantitatively react with one another to produce salt and water molecules. The reaction between phosphoric acid and potassium hydroxide will produce the salt potassium phosphate and water.
Complete step by step answer:
The reaction given in the above question is a neutralization reaction. In a neutralization reaction, the acid and the base quantitatively react with one another to produce salt and water molecules.
$acid + base\longrightarrow salt + water$
If a strong acid and strong base react with each other such that there is complete neutralization, the pH of the resulting solution will be equal to 7. On the other hand, the neutralization of a strong acid and weak base will give a pH of less than 7. Similarly the neutralization of a strong base and a weak acid will give a pH of greater than 7.
Since phosphoric acid is a weak acid and potassium hydroxide is a strong base, the pH at the neutralization point will be slightly greater than 7.
To determine how many moles of potassium hydroxide will react with one mole of phosphoric acid, we need to find out the number of replaceable protons present in phosphoric acid. This can be done by looking at its structure:
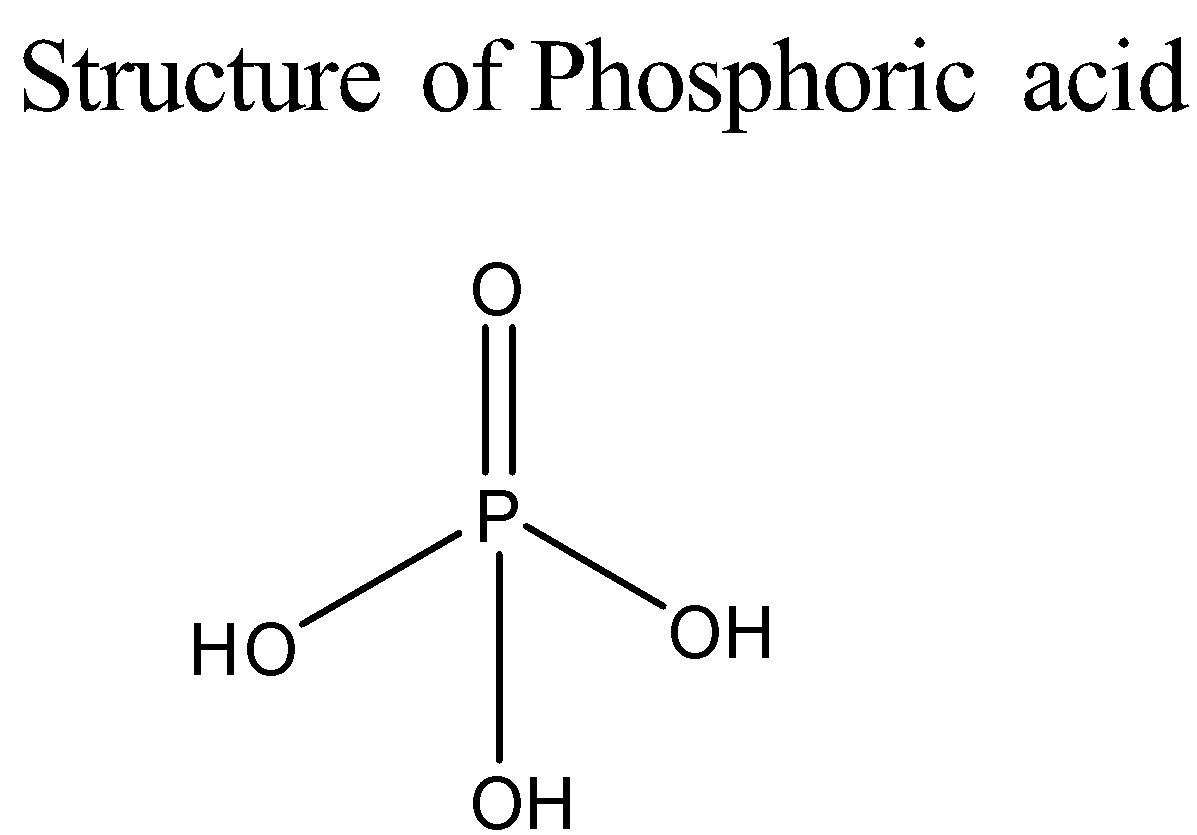
Only those hydrogen atoms that are directly bonded to oxygen atoms present in the hydroxyl groups are replaceable protons. Hence for neutralizing one mole of phosphoric acid, we would need three moles of potassium hydroxide. The reaction will produce the salt potassium phosphate and three moles of water. The reaction is given below:
$ { H }_{ 3 }{ PO }_{ 4 }(aq)+3KOH(aq)\rightarrow { K }_{ 3 }{ PO }_{ 4 }(aq)+3{ H }_{ 2 }O(l)$
Now, one mole of a substance has $6.022\times { 10 }^{ 23 }$ (Avogadro number) particles. These particles can be atoms, molecules, ions, or electrons.
The neutralization reaction between one mole of phosphoric acid and three moles of potassium hydroxide gives three moles of water.
1 mole of water contains = $ 6.022\times { 10 }^{ 23 }$ water molecules
Therefore, 3 moles of water will have = $ \cfrac { (6.022\times { 10 }^{ 23 })\quad molecules }{ 1\quad mol } \times 3\quad mol=1.81\times { 10 }^{ 24 }\quad molecules$
Hence $1.81\times { 10 }^{ 24 }$ water molecules will be produced on complete neutralization between one mole of phosphoric acid and three moles of potassium hydroxide.
Note: The number of replaceable protons present in an acid is also called its n-factor. The n-factor or the valence factor for a species is not a fixed quantity. It changes with the type of reaction, the products of the reaction so be careful while calculating the n-factor for a particular species.
Complete step by step answer:
The reaction given in the above question is a neutralization reaction. In a neutralization reaction, the acid and the base quantitatively react with one another to produce salt and water molecules.
$acid + base\longrightarrow salt + water$
If a strong acid and strong base react with each other such that there is complete neutralization, the pH of the resulting solution will be equal to 7. On the other hand, the neutralization of a strong acid and weak base will give a pH of less than 7. Similarly the neutralization of a strong base and a weak acid will give a pH of greater than 7.
Since phosphoric acid is a weak acid and potassium hydroxide is a strong base, the pH at the neutralization point will be slightly greater than 7.
To determine how many moles of potassium hydroxide will react with one mole of phosphoric acid, we need to find out the number of replaceable protons present in phosphoric acid. This can be done by looking at its structure:

Only those hydrogen atoms that are directly bonded to oxygen atoms present in the hydroxyl groups are replaceable protons. Hence for neutralizing one mole of phosphoric acid, we would need three moles of potassium hydroxide. The reaction will produce the salt potassium phosphate and three moles of water. The reaction is given below:
$ { H }_{ 3 }{ PO }_{ 4 }(aq)+3KOH(aq)\rightarrow { K }_{ 3 }{ PO }_{ 4 }(aq)+3{ H }_{ 2 }O(l)$
Now, one mole of a substance has $6.022\times { 10 }^{ 23 }$ (Avogadro number) particles. These particles can be atoms, molecules, ions, or electrons.
The neutralization reaction between one mole of phosphoric acid and three moles of potassium hydroxide gives three moles of water.
1 mole of water contains = $ 6.022\times { 10 }^{ 23 }$ water molecules
Therefore, 3 moles of water will have = $ \cfrac { (6.022\times { 10 }^{ 23 })\quad molecules }{ 1\quad mol } \times 3\quad mol=1.81\times { 10 }^{ 24 }\quad molecules$
Hence $1.81\times { 10 }^{ 24 }$ water molecules will be produced on complete neutralization between one mole of phosphoric acid and three moles of potassium hydroxide.
Note: The number of replaceable protons present in an acid is also called its n-factor. The n-factor or the valence factor for a species is not a fixed quantity. It changes with the type of reaction, the products of the reaction so be careful while calculating the n-factor for a particular species.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




