
Upon treatment with ${{I}_{2}}$ and aqueous $NaOH$, which of the following compounds will form iodoform?
Answer
587.7k+ views
Hint: The iodoform reaction is answered by some ketones and few secondary alcohols. The reaction is confirmed by the change in color of the solution due to the formation of $CH{{I}_{3}}$ which is the organic compound iodoform.
Complete step-by-step answer:
Iodoform test is used to check the presence of few of the many carbonyl compounds as well as secondary alcohols in a given solution of unknown compounds.
The reaction of iodine, a base and methyl ketone under consideration gives a yellow precipitate along with the characteristic antiseptic smell. The test is answered by a few secondary alcohols that contain at least one methyl group in the alpha position.
The yellow colour of the solution is due to the formation of triiodomethane.
Compounds that give positive iodoform test are :
- Acetaldehyde
- Methyl Ketones
- Ethanol
- Secondary alcohols containing methyl groups in alpha position
It is important to know that 3 moles of iodine along with 4 moles of the strong base is used in the complete reaction process. An example is given below depicting the reaction mechanism:
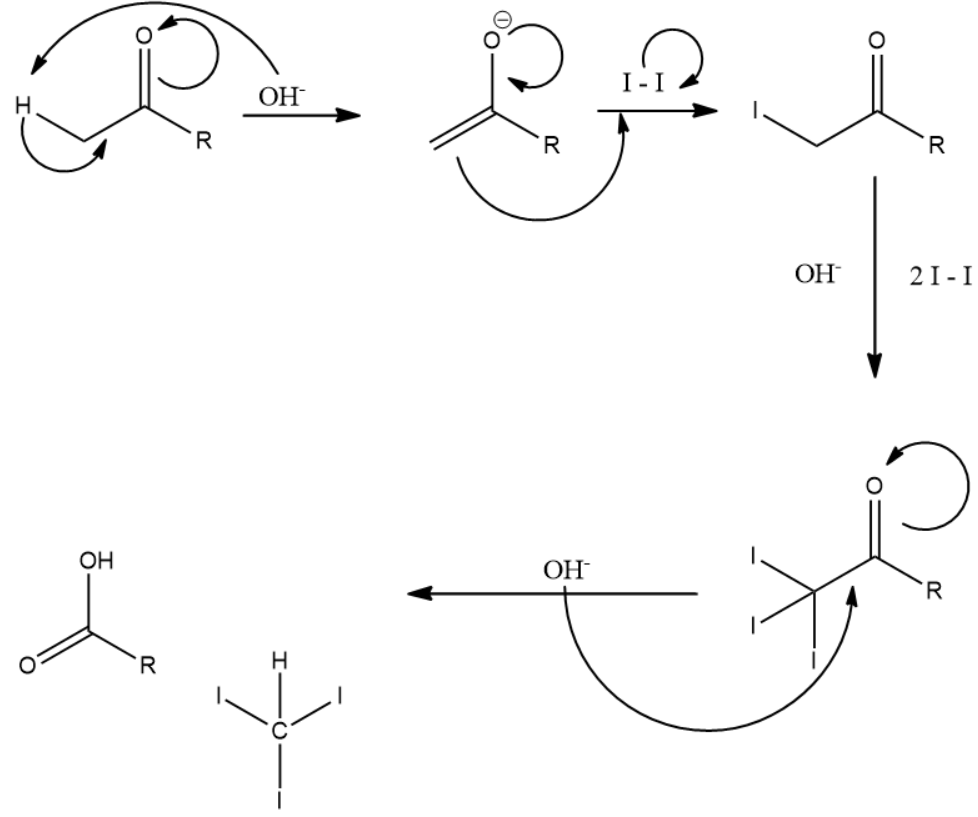
From the above reaction mechanism and explanation, we will now identify the compounds that will answer the iodoform test.
From the options mentioned above, the compound that will test positive for iodoform test is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CH(OH)C{{H}_{3}}$.
Therefore, the correct answer is option (D).
Note: In the above explanation of the iodoform test we mentioned that 4 moles of base are used up. However only moles of base are indicated in the figure above. This is because the fourth mole of base is used to convert the acid into salt of the carboxylic acid which is formed along with triiodomethane as a product.
Complete step-by-step answer:
Iodoform test is used to check the presence of few of the many carbonyl compounds as well as secondary alcohols in a given solution of unknown compounds.
The reaction of iodine, a base and methyl ketone under consideration gives a yellow precipitate along with the characteristic antiseptic smell. The test is answered by a few secondary alcohols that contain at least one methyl group in the alpha position.
The yellow colour of the solution is due to the formation of triiodomethane.
Compounds that give positive iodoform test are :
- Acetaldehyde
- Methyl Ketones
- Ethanol
- Secondary alcohols containing methyl groups in alpha position
It is important to know that 3 moles of iodine along with 4 moles of the strong base is used in the complete reaction process. An example is given below depicting the reaction mechanism:

From the above reaction mechanism and explanation, we will now identify the compounds that will answer the iodoform test.
From the options mentioned above, the compound that will test positive for iodoform test is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CH(OH)C{{H}_{3}}$.
Therefore, the correct answer is option (D).
Note: In the above explanation of the iodoform test we mentioned that 4 moles of base are used up. However only moles of base are indicated in the figure above. This is because the fourth mole of base is used to convert the acid into salt of the carboxylic acid which is formed along with triiodomethane as a product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




