
Ullmann reaction is given by:
(A) $ {C_6}{H_5} - Cl $
(B)
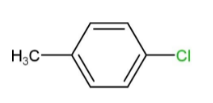
(C)
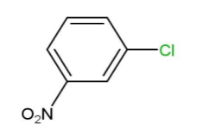
(D)
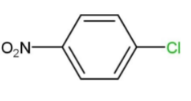



Answer
517.5k+ views
Hint: We know that the Ullmann reaction, also known as Ullmann coupling is an organic reaction in which two aryl halides are coupled in the presence of copper to produce a bi aryl as a result. Fritz Ullmann, a German chemist, is the name given to this coupling reaction.
Complete answer:
Step 1: The Ullmann reaction entails the creation of an active copper(I) species when an excess of metallic copper is introduced to the aryl halide at moderately high temperatures, that is >200 degree Celsius.
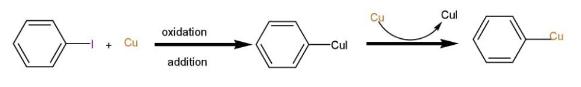
Step 2: Another haloarene molecule is oxidatively added to this copper(I) species, forming a bond between the two molecules
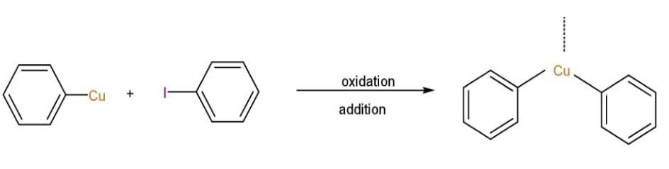
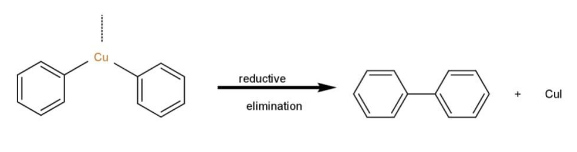
Step 3: The copper compound formed by the two aryl halide molecules is reductively eliminated in the final step of the Ullmann reaction process, resulting in the forming of a new carbon-carbon bond between the two aryl compounds.
That is, ortho and para nitro derivatives of halo benzene, as well as those halo benzenes with deactivating groups at the ortho and nitro sites, are used in Ullmann's reaction. As a result, Option "D" is the right solution.
Note:
Applications of the Ullmann Reaction are:
1. Ullmann Reaction Applications Biphenylenes can be made from 2,2-iodophenyl using the Ullmann reaction.
2. This reaction can be used to close five-membered rings as well.
3. When one of the reactants is provided in abundance, an unsymmetrical reaction can be achieved.
4. This reaction will couple chiral reactants into chiral products.
Complete answer:
Step 1: The Ullmann reaction entails the creation of an active copper(I) species when an excess of metallic copper is introduced to the aryl halide at moderately high temperatures, that is >200 degree Celsius.

Step 2: Another haloarene molecule is oxidatively added to this copper(I) species, forming a bond between the two molecules

Step 3: The copper compound formed by the two aryl halide molecules is reductively eliminated in the final step of the Ullmann reaction process, resulting in the forming of a new carbon-carbon bond between the two aryl compounds.

That is, ortho and para nitro derivatives of halo benzene, as well as those halo benzenes with deactivating groups at the ortho and nitro sites, are used in Ullmann's reaction. As a result, Option "D" is the right solution.
Note:
Applications of the Ullmann Reaction are:
1. Ullmann Reaction Applications Biphenylenes can be made from 2,2-iodophenyl using the Ullmann reaction.
2. This reaction can be used to close five-membered rings as well.
3. When one of the reactants is provided in abundance, an unsymmetrical reaction can be achieved.
4. This reaction will couple chiral reactants into chiral products.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




