
True statement is:
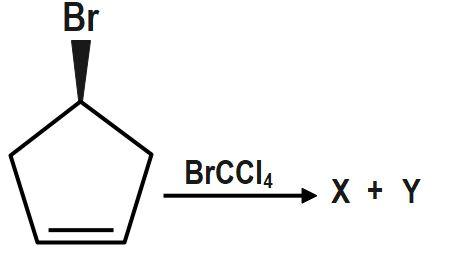
(A) X and Y both are optically active.
(B) X and Y both are enantiomers of each other.
(C) X and Y both are optically inactive.
(D) One is active and the other is optically inactive.

Answer
517.2k+ views
Hint: We know that the isomers which can rotate the plane of polarized light are known as the optically active complexes. The essential condition for the complex to exhibit the optical activity is that the isomer should not possess the plane of symmetry in the structure.
Complete answer:
If the plane divides the complex into the equal half then the complex is optically inactive. However, if not then the complex is said to be optically active. The bidentate ligand donates its two electrons and forms a bond with the metal. There is a certain substance that can rotate the plane polarized light. These are called the optically active substances.
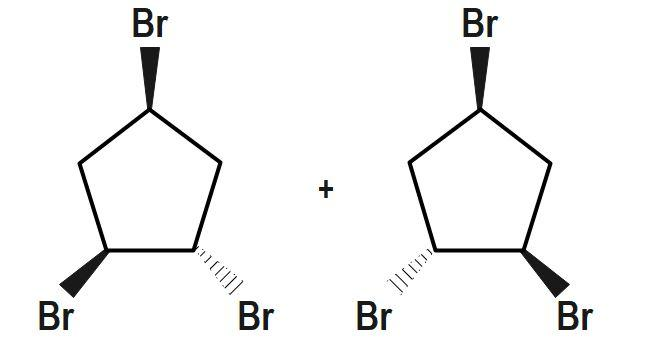
The isomers which rotate the plane of polarized light equally but in opposite directions are called the optically active isomers. The essential condition for a substance to show optical activity is that the substance should not have a plane of symmetry in its structure. The optical isomers have identical physical and chemical properties. They differ only in the direction in which they rotate the plane of polarized light.
Therefore, the correct answer is option A.
Note:
Remember that the similarities between a racemic mixture and meso compound are that they both are organic compounds and are optically inactive. The main difference between them is that a racemic mixture contains non-identical isomers while a meso compound contains an identical isomer.
Complete answer:
If the plane divides the complex into the equal half then the complex is optically inactive. However, if not then the complex is said to be optically active. The bidentate ligand donates its two electrons and forms a bond with the metal. There is a certain substance that can rotate the plane polarized light. These are called the optically active substances.

The isomers which rotate the plane of polarized light equally but in opposite directions are called the optically active isomers. The essential condition for a substance to show optical activity is that the substance should not have a plane of symmetry in its structure. The optical isomers have identical physical and chemical properties. They differ only in the direction in which they rotate the plane of polarized light.
Therefore, the correct answer is option A.
Note:
Remember that the similarities between a racemic mixture and meso compound are that they both are organic compounds and are optically inactive. The main difference between them is that a racemic mixture contains non-identical isomers while a meso compound contains an identical isomer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




