
To which of the following species, the octet rule is not applicable?
This question has multiple correct options
A.$Br{F_5}$
B.$S{F_6}$
C.$I{F_7}$
D.$C{O_2}$
Answer
577.2k+ views
Hint:We can define transfer of electrons as a process in which an electron shares one or more electrons to its neighboring atom. We know that there must be eight electrons in the valence electron orbital of an atom. This is called an octet rule. If an atom has less than eight electrons, they tend to react and yield stable compounds.
Complete step by step answer:
We can state octet rule that an atom is more stable when their valence electron shells are occupied with eight electrons. Molecules like halogens, oxygen, nitrogen and carbon does not violate the octet rule. All the elements that are present in the main group of the periodic table obey the octet rule.
We can say a stable arrangement is reached when the atom is enclosed by eight electrons. This octet could be made up of its own electrons and some electrons that are shared. Therefore, an atom continues to form bonds until an octet of electrons is made.
Let us now calculate the total number of electrons present in all compounds by adding the electrons in bond pairs and lone pairs.
-The number of electrons in $Br{F_5}$ is ten electrons. We can draw the structure of this compound as,
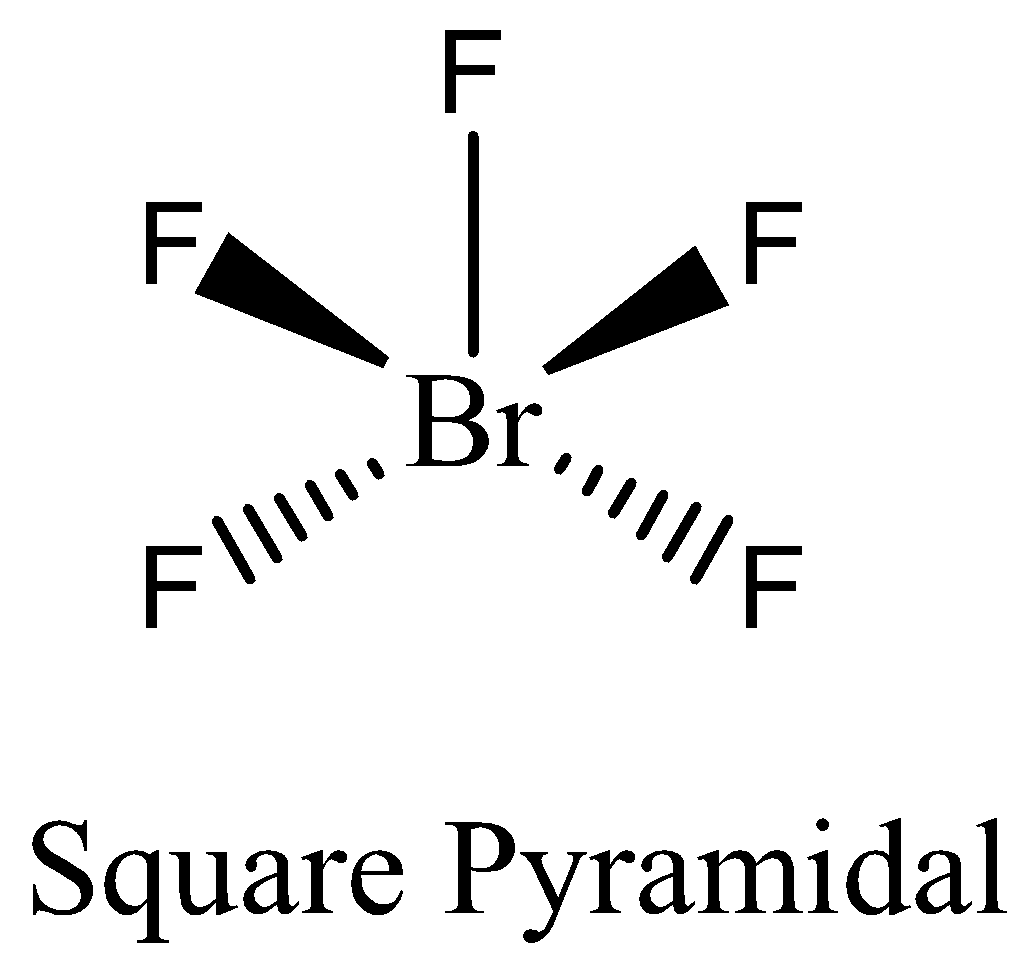
-In $Br{F_5}$ the atom that violates octet rule is bromine. The central bromine atom forms five covalent bonds to five fluorine atoms, therefore it is an expanded valence shell molecule. The atom of bromine expands its octet, hence the molecule $Br{F_5}$ violates the octet rule.
Therefore, the option (A) is correct.
-The number of electrons in $S{F_6}$ is twelve electrons. We can draw the structure of this compound as,
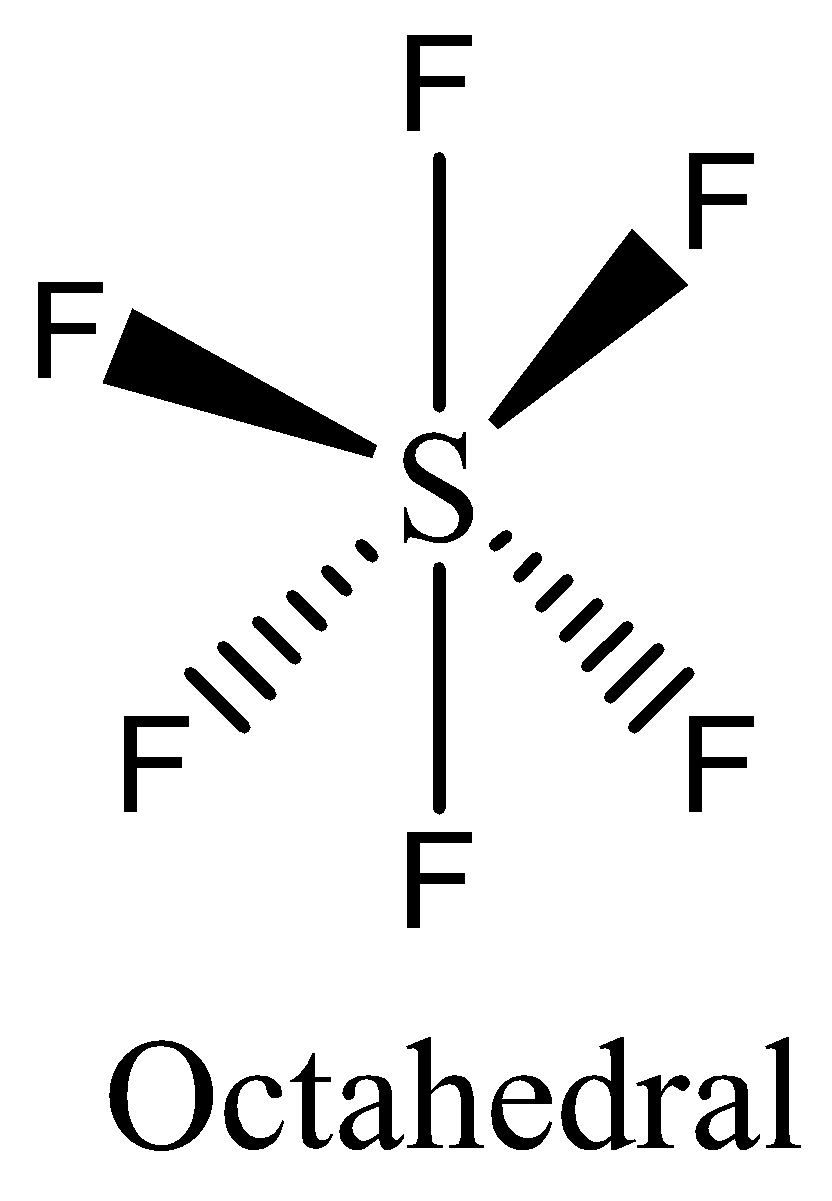
-In $S{F_6}$ the atom that violates octet rule is sulfur. The central sulfur atom forms six covalent bonds to six fluorine atoms, therefore it is an expanded valence shell molecule. The atom of sulfur expands its octet, hence the molecule $S{F_6}$ violates the octet rule. Option (B) is correct.
The number of electrons in $I{F_7}$ is fourteen electrons. We can draw the structure of this compound as,
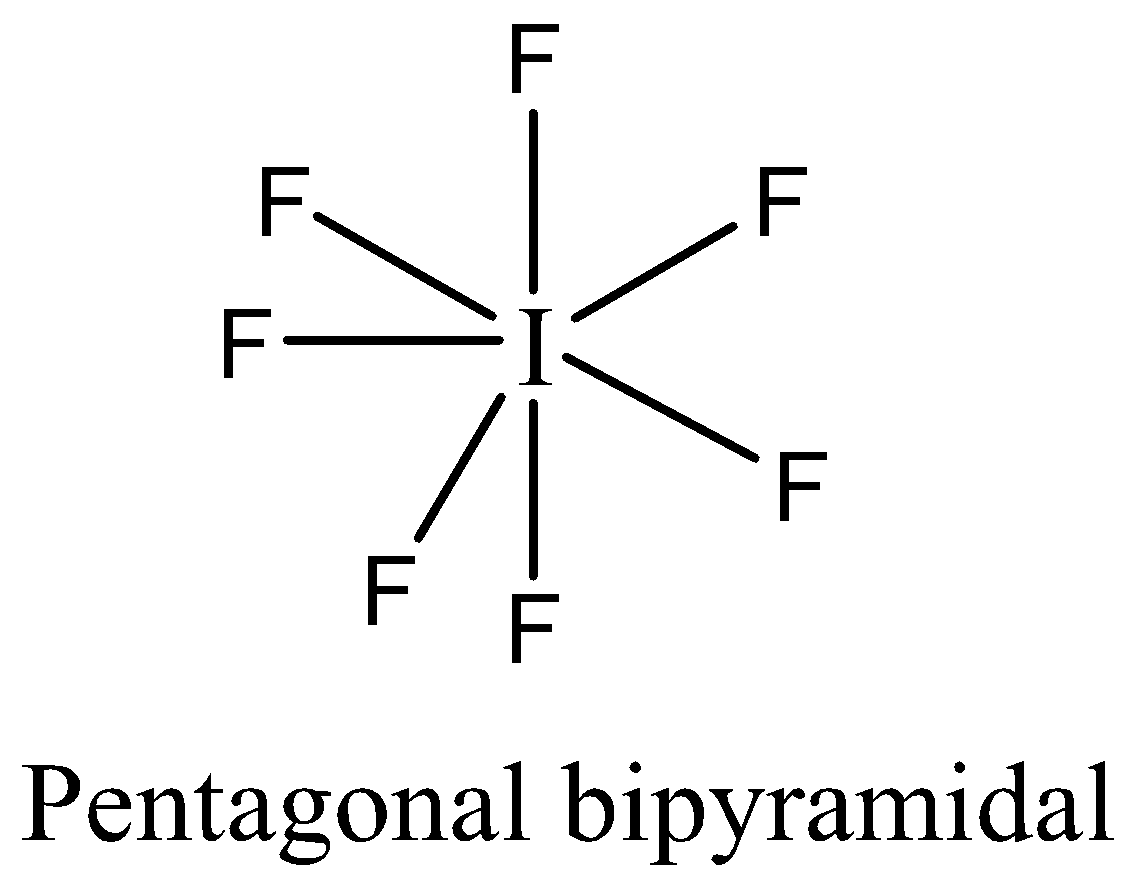
-In $I{F_7}$ the atom that violates octet rule is iodine. The central iodine atom forms seven covalent bonds to seven fluorine atoms, therefore, it is an expanded valence shell molecule. The atom of iodine expands its octet, hence the molecule $I{F_7}$ violates the octet rule.
Therefore, the option (C) is correct.
-The number of electrons in $C{O_2}$ is eight. We can draw the structure of this compound as,
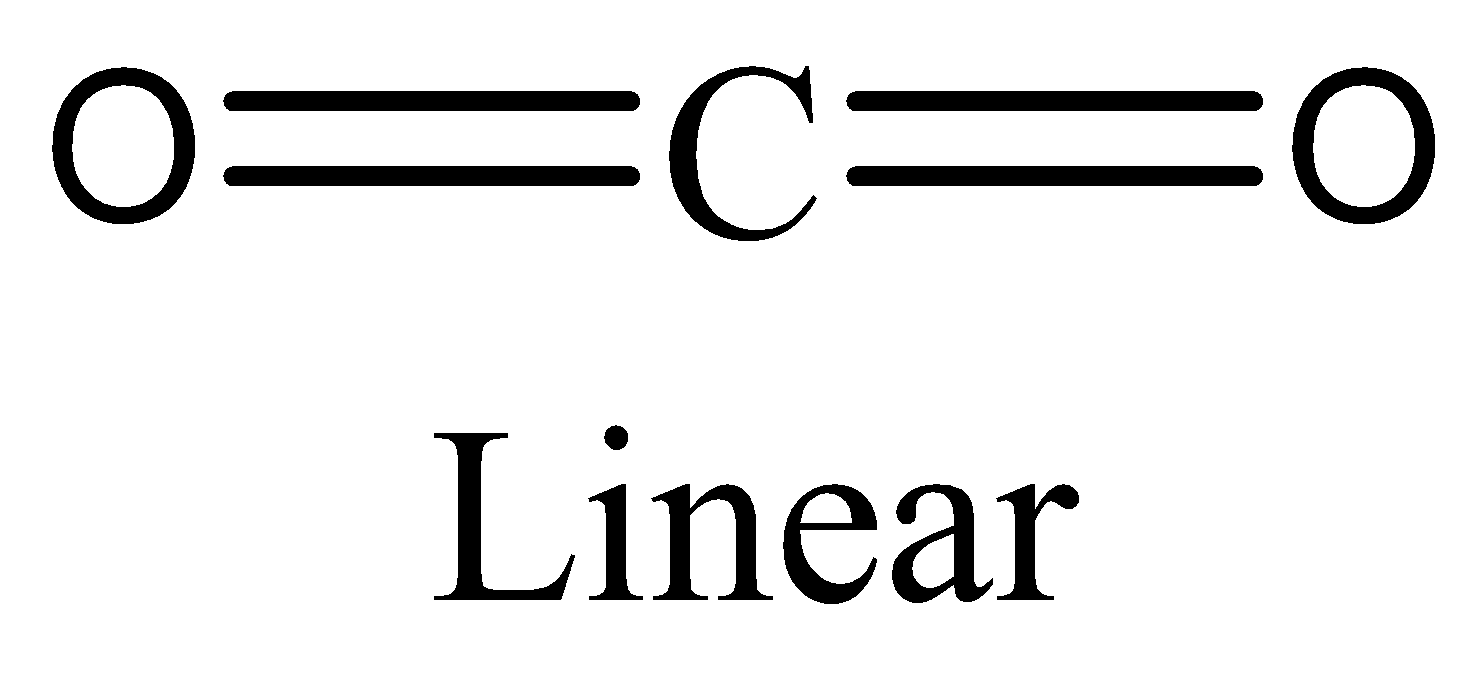
This compound obeys octet rule. Therefore, the option (D) is incorrect.
Thus option A,B and C is correct.
Note:
Some of the compounds that do not obey octet rule are hydrogen, phosphorus, sulfur, lithium. An example of a compound that violates octet rule is $B{H_3}$.
In $B{H_3}$ the atom that deviates octet rule is boron. We can draw the structure of this compound as,
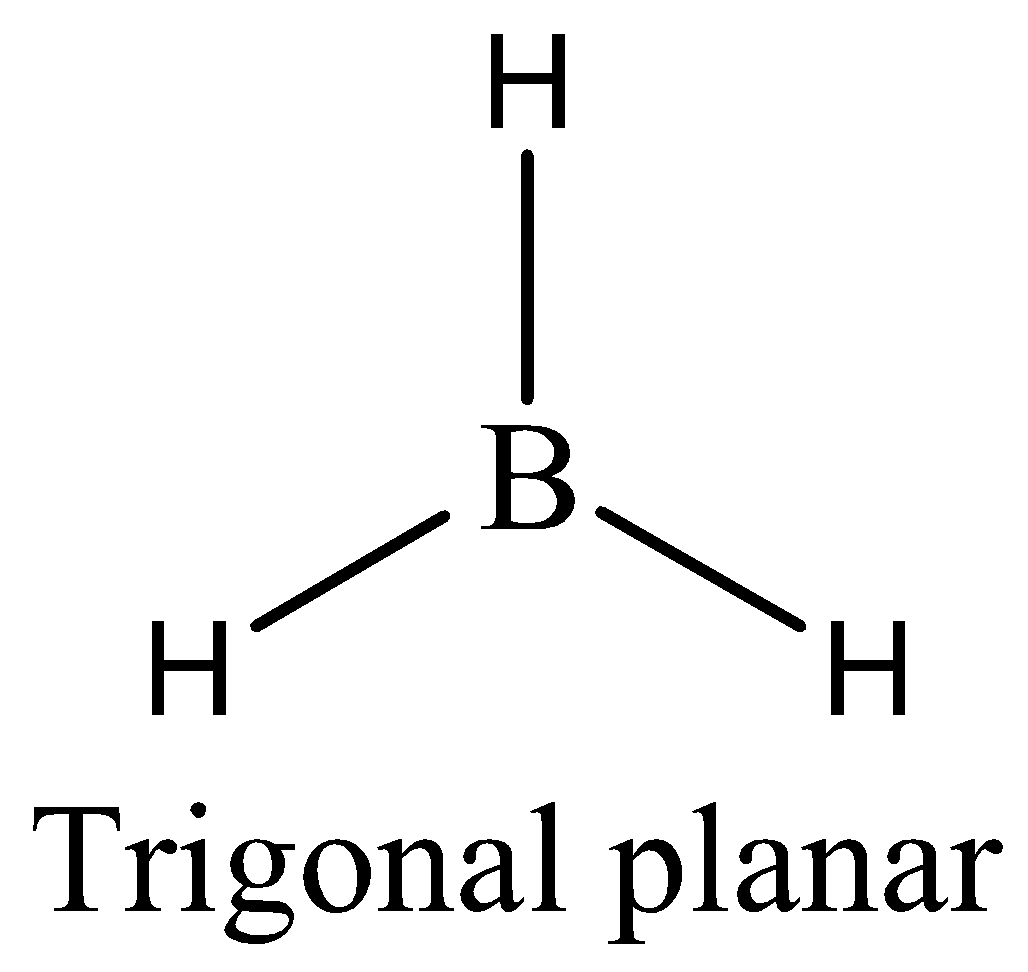
There are only six outermost electrons in $B{H_3}$ around the central atom boron. The atom of boron has incomplete octet and hence, the molecule $B{H_3}$ violates the octet rule.
Complete step by step answer:
We can state octet rule that an atom is more stable when their valence electron shells are occupied with eight electrons. Molecules like halogens, oxygen, nitrogen and carbon does not violate the octet rule. All the elements that are present in the main group of the periodic table obey the octet rule.
We can say a stable arrangement is reached when the atom is enclosed by eight electrons. This octet could be made up of its own electrons and some electrons that are shared. Therefore, an atom continues to form bonds until an octet of electrons is made.
Let us now calculate the total number of electrons present in all compounds by adding the electrons in bond pairs and lone pairs.
-The number of electrons in $Br{F_5}$ is ten electrons. We can draw the structure of this compound as,

-In $Br{F_5}$ the atom that violates octet rule is bromine. The central bromine atom forms five covalent bonds to five fluorine atoms, therefore it is an expanded valence shell molecule. The atom of bromine expands its octet, hence the molecule $Br{F_5}$ violates the octet rule.
Therefore, the option (A) is correct.
-The number of electrons in $S{F_6}$ is twelve electrons. We can draw the structure of this compound as,

-In $S{F_6}$ the atom that violates octet rule is sulfur. The central sulfur atom forms six covalent bonds to six fluorine atoms, therefore it is an expanded valence shell molecule. The atom of sulfur expands its octet, hence the molecule $S{F_6}$ violates the octet rule. Option (B) is correct.
The number of electrons in $I{F_7}$ is fourteen electrons. We can draw the structure of this compound as,

-In $I{F_7}$ the atom that violates octet rule is iodine. The central iodine atom forms seven covalent bonds to seven fluorine atoms, therefore, it is an expanded valence shell molecule. The atom of iodine expands its octet, hence the molecule $I{F_7}$ violates the octet rule.
Therefore, the option (C) is correct.
-The number of electrons in $C{O_2}$ is eight. We can draw the structure of this compound as,
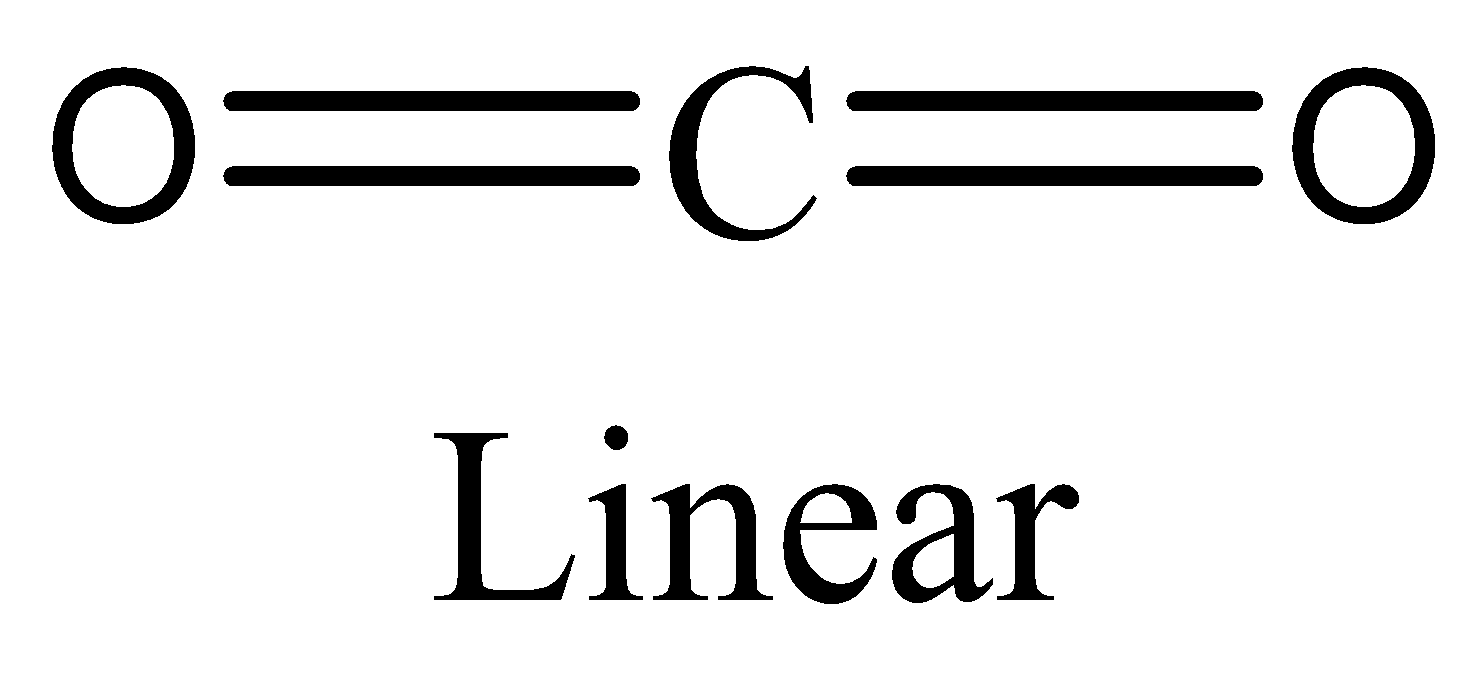
This compound obeys octet rule. Therefore, the option (D) is incorrect.
Thus option A,B and C is correct.
Note:
Some of the compounds that do not obey octet rule are hydrogen, phosphorus, sulfur, lithium. An example of a compound that violates octet rule is $B{H_3}$.
In $B{H_3}$ the atom that deviates octet rule is boron. We can draw the structure of this compound as,

There are only six outermost electrons in $B{H_3}$ around the central atom boron. The atom of boron has incomplete octet and hence, the molecule $B{H_3}$ violates the octet rule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




