
Three types of bands are shown in the figure given below showing the position of the valence band and conduction band. The figure A, B and C represents:
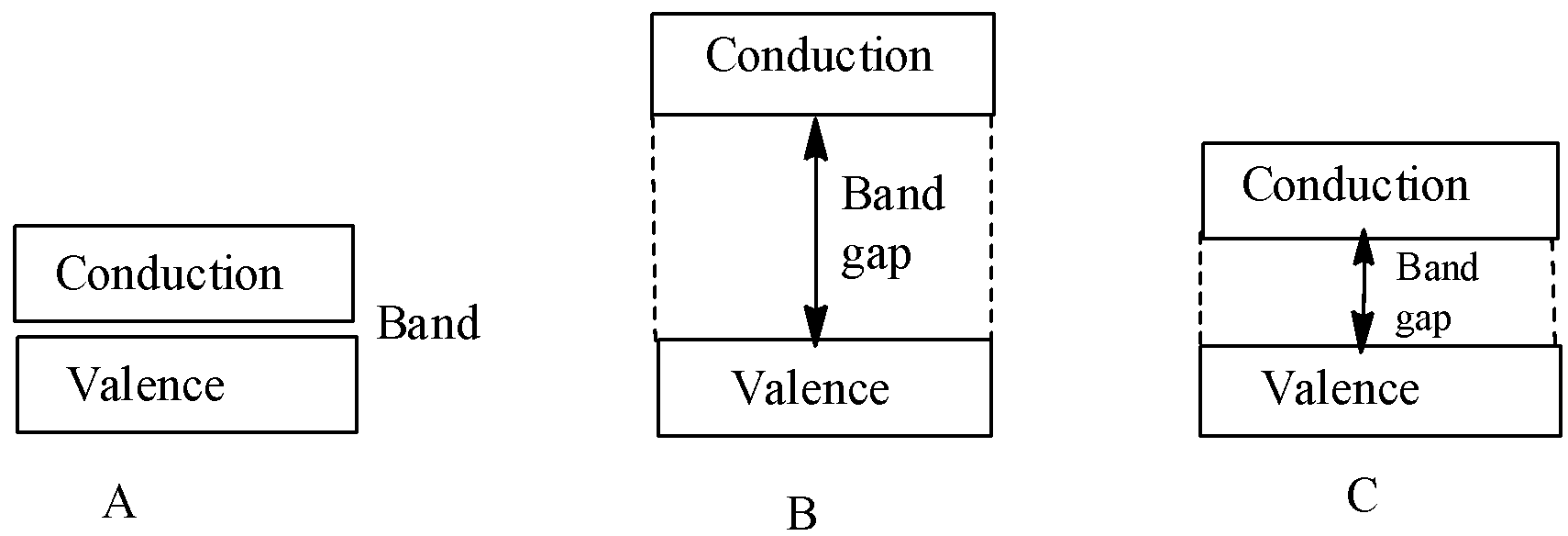
(A) (i) Non-metal (ii) Metal (iii) Semiconductor
(B) (i) Semiconductor (ii) Insulator (iii) Conductor
(C) (i) Metal (ii) Insulator (iii) Semiconductor
(D) (i) Insulator (ii) Conductor (iii) Semiconductor

Answer
561.3k+ views
Hint Energy band theory is based on Pauli’s exclusion principle. Band gap energy is the energy gap between conduction band and valence band and width of band gap depends upon the nature of substance.
Complete answer:
In the classes of chemistry, we have studied the topic of band theory and related definitions. Let us see it in detail now.
Solids are classified into conductor, semiconductor and insulator according to the energy band theory.
- In conductor conduction band and valence band are overlapped so there is no bond gap in between them. Due to the large number of electrons in the valence band they show high conductivity and therefore its resistivity is low. All the metal atoms are examples of conductors.
- In insulators the energy gap is very large, so conduction of electrons from the valence band to conduction band is not possible. They have high resistance and are poor conductors of electricity.
- In semiconductor small band gap energy exists. So, due to small energy band gaps some electrons can be thermally excited to the conduction band. These thermally excited electrons can move in a conduction band and can conduct current.
- So, in the given figure ‘A’ represents the conductor or metal, ‘B’ represents the insulators or non-metals and ‘C’ represents the semiconductors.
So, option (C) is the correct option.
Note: The band gap in solids is relatively smallest in semiconductors while it is very large in insulators. So on increasing temperature conductivity of semiconductors increases while conductivity of insulators remains unchanged.
Complete answer:
In the classes of chemistry, we have studied the topic of band theory and related definitions. Let us see it in detail now.
Solids are classified into conductor, semiconductor and insulator according to the energy band theory.
- In conductor conduction band and valence band are overlapped so there is no bond gap in between them. Due to the large number of electrons in the valence band they show high conductivity and therefore its resistivity is low. All the metal atoms are examples of conductors.
- In insulators the energy gap is very large, so conduction of electrons from the valence band to conduction band is not possible. They have high resistance and are poor conductors of electricity.
- In semiconductor small band gap energy exists. So, due to small energy band gaps some electrons can be thermally excited to the conduction band. These thermally excited electrons can move in a conduction band and can conduct current.
- So, in the given figure ‘A’ represents the conductor or metal, ‘B’ represents the insulators or non-metals and ‘C’ represents the semiconductors.
So, option (C) is the correct option.
Note: The band gap in solids is relatively smallest in semiconductors while it is very large in insulators. So on increasing temperature conductivity of semiconductors increases while conductivity of insulators remains unchanged.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




