
The use of aspartame is limited to cold foods and drinks because:
A. it is unstable to heat and decomposes at cooking temperature
B. it is 500 times sweeter than cane sugar
C. it becomes bitter at cooking temperature
D. it reacts with the food at cooking temperature
Answer
592.2k+ views
Hint: Aspartame is an example of artificial sweetening agent. Such substances are added to increase shelf life of stored food or cosmetic purposes. These agents do not have any nutritional value. These substances have high sweetness in comparison to cane sugar but low calories.
Complete answer:
Let us discuss the structure of aspartame and its properties to understand the reason for this question;
Aspartame: It is a methyl ester of the dipeptide of the natural amino acids which are L-aspartic acid and L-phenylalanine.
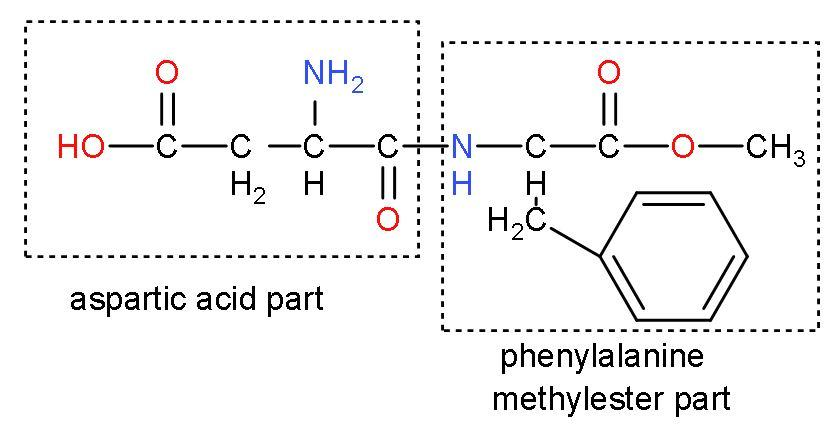
- Under acidic or alkaline conditions, aspartame generates methanol by hydrolysis. Under more conditions, the peptide bonds are also hydrolyzed, resulting in free amino acids.
- Aspartame is 100 times as sweet as sucrose. As aspartame produces 17 kJ of energy per gram when metabolised. The amount of aspartame needed to produce a sweet taste is so small that its caloric count is almost negligible.
- Aspartame decomposes at cooking on high temperature.
The correct answer to this question is option ‘a’ which says that use of aspartame is limited to cold foods and soft drinks because it is unstable at cooking temperature.
So, the correct answer is “Option A”.
Additional Information:
(1)- Use of aspartame has severe effects and causes risk of headaches, depression, dizziness and attention deficit hyperactivity disorder (ADHD).
(2) Aspartame is denoted as E951. Aspartame stimulates the taste buds on the tongue as it is a sugar substitute. It is used in a variety of desserts, sweets, foods, beverages, chewing gums and weight-control products.
(3) Artificial sweetener is associated with long-term weight gain and increased risk of diabetes, high blood pressure and heart disease. Consumption of aspartame is increasing.
Note: The structure of aspartame is almost similar to sucralose, so do not get confused between the properties of the two. Sucralose is a trichloro derivative of sucrose. It tastes like sugar. It is stable at cooking temperature and it does not provide calories.
Complete answer:
Let us discuss the structure of aspartame and its properties to understand the reason for this question;
Aspartame: It is a methyl ester of the dipeptide of the natural amino acids which are L-aspartic acid and L-phenylalanine.

- Under acidic or alkaline conditions, aspartame generates methanol by hydrolysis. Under more conditions, the peptide bonds are also hydrolyzed, resulting in free amino acids.
- Aspartame is 100 times as sweet as sucrose. As aspartame produces 17 kJ of energy per gram when metabolised. The amount of aspartame needed to produce a sweet taste is so small that its caloric count is almost negligible.
- Aspartame decomposes at cooking on high temperature.
The correct answer to this question is option ‘a’ which says that use of aspartame is limited to cold foods and soft drinks because it is unstable at cooking temperature.
So, the correct answer is “Option A”.
Additional Information:
(1)- Use of aspartame has severe effects and causes risk of headaches, depression, dizziness and attention deficit hyperactivity disorder (ADHD).
(2) Aspartame is denoted as E951. Aspartame stimulates the taste buds on the tongue as it is a sugar substitute. It is used in a variety of desserts, sweets, foods, beverages, chewing gums and weight-control products.
(3) Artificial sweetener is associated with long-term weight gain and increased risk of diabetes, high blood pressure and heart disease. Consumption of aspartame is increasing.
Note: The structure of aspartame is almost similar to sucralose, so do not get confused between the properties of the two. Sucralose is a trichloro derivative of sucrose. It tastes like sugar. It is stable at cooking temperature and it does not provide calories.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




