
The type of ion found in potassium phosphates is:
[A] ${{X}^{+}}$
[B] ${{X}^{2+}}$
[C] ${{X}^{3+}}$
[D] $X{{O}_{3}}^{2-}$
[E] $X{{O}_{4}}^{3-}$
Answer
592.2k+ views
Hint: This question has multiple correct answers. One answer will be for the cation and the other for anion. A phosphate ion consists of 4 oxygen atoms, one forms a double bond with phosphorus whereas the others are negative ions.
Complete answer:
We use the term potassium phosphate to describe some general forms of salts formed by potassium and phosphate ions.
We know that phosphate ion is $P{{O}_{4}}^{3-}$. One oxygen atom forms a double bond with the potassium atom and the other oxygen atoms stay as negatively charged ions thus, it has a negative charge of 3. We can draw the structure of phosphate as ion as-
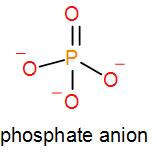
Phosphate combined with potassium gives us 3 kinds of compounds namely mono potassium phosphate, di-potassium phosphate and tripotassium phosphate.
As we can understand from their names itself monophosphate contains one potassium ion whereas di- and tri- potassium phosphates will contain 2 and 3 potassium ions respectively.
In mono potassium phosphate, di-potassium phosphate and tripotassium phosphate respectively one, two and three hydrogen atoms are replaced by potassium ions. Now we can draw their structures as-
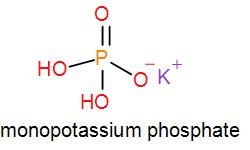
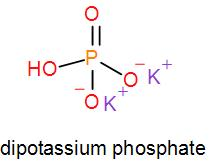
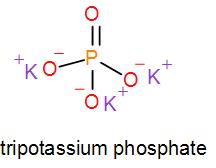
As we can see from the above structures that a potassium cation is found in all the structures therefore, the type of cation found in potassium phosphates is ${{K}^{+}}$ which is in the form ${{X}^{+}}$. Therefore, option [A] is one of the correct answers.
Similarly, we can see that it has phosphate ions which is $P{{O}_{4}}^{3-}$ and it is of the type $X{{O}_{4}}^{3-}$. So, option [D] is also a correct option.
Therefore, the correct answer is option [A] ${{X}^{+}}$ and option [D] $X{{O}_{4}}^{3-}$.
So, the correct answer is “Option A and D”.
Note: We use mono-potassium phosphate together with di-potassium phosphate, a fertilizer, food additive and also as a buffering agent. Tri-potassium phosphate is used as a strong base. Phosphates salts are also used in the medicine industry.
Complete answer:
We use the term potassium phosphate to describe some general forms of salts formed by potassium and phosphate ions.
We know that phosphate ion is $P{{O}_{4}}^{3-}$. One oxygen atom forms a double bond with the potassium atom and the other oxygen atoms stay as negatively charged ions thus, it has a negative charge of 3. We can draw the structure of phosphate as ion as-


Phosphate combined with potassium gives us 3 kinds of compounds namely mono potassium phosphate, di-potassium phosphate and tripotassium phosphate.
As we can understand from their names itself monophosphate contains one potassium ion whereas di- and tri- potassium phosphates will contain 2 and 3 potassium ions respectively.
In mono potassium phosphate, di-potassium phosphate and tripotassium phosphate respectively one, two and three hydrogen atoms are replaced by potassium ions. Now we can draw their structures as-

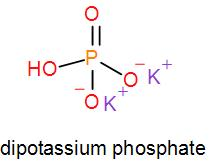
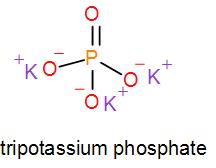
As we can see from the above structures that a potassium cation is found in all the structures therefore, the type of cation found in potassium phosphates is ${{K}^{+}}$ which is in the form ${{X}^{+}}$. Therefore, option [A] is one of the correct answers.
Similarly, we can see that it has phosphate ions which is $P{{O}_{4}}^{3-}$ and it is of the type $X{{O}_{4}}^{3-}$. So, option [D] is also a correct option.
Therefore, the correct answer is option [A] ${{X}^{+}}$ and option [D] $X{{O}_{4}}^{3-}$.
So, the correct answer is “Option A and D”.
Note: We use mono-potassium phosphate together with di-potassium phosphate, a fertilizer, food additive and also as a buffering agent. Tri-potassium phosphate is used as a strong base. Phosphates salts are also used in the medicine industry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




