
The type of hybridization of boron in diborane is?
A- $sp$ – hybridization
B- $sp^{ 3 }$ – hybridization
C- $sp^{ 2 }$ – hybridization
D- ${ sp }^{ 3 }{ d }^{ 2 }$- hybridization
Answer
599.4k+ views
Hint: Try to figure out hybridization based on the number of neighboring atoms around boron atoms. Diborane consists of special bonds to satisfy the octet configuration of boron atoms.
Complete step by step answer:
- We know that diborane consists of 2 boron atoms and 6 hydrogen atoms. Out of these 6 hydrogen atoms 4 hydrogen atoms are attached to only one boron atom remaining two hydrogen atoms are attached to both borons through a special type of bond.
-Boron has 3 electrons in valence shell; it cannot complete octet directly through covalent bonds. In the excited state of boron, it has 3 unpaired electrons with 4 orbitals which can involve in bonding. In diborane 2 hydrogen atoms in between two boron atoms are connected to both atoms through a special 3 centered 2 electron bond. In 3 centered 2 electron bonds, 2 electrons are shared in between 3 centers; those are 2 boron atoms and 1 hydrogen atom to satisfy the octet of boron. This type of bond is also considered as regular while calculating hybridization. Since each boron atom has 4 bonds so hybridization will $sp^{ 3 }$ – hybridization.
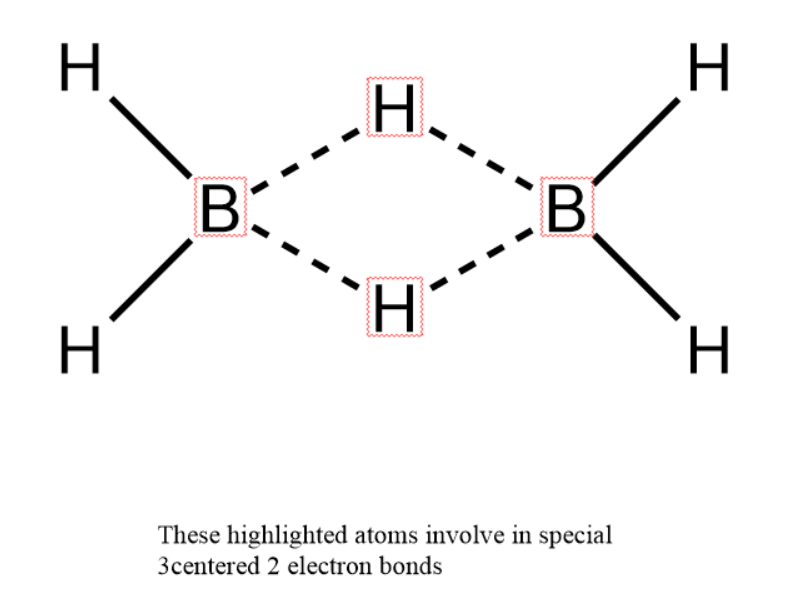
Therefore, option B is correct.
Note:
has only 3 electrons in the outer shell and can form only 3 hybridized orbitals. So both boron atoms are connected whether it is through direct or indirect bonding.
Complete step by step answer:
- We know that diborane consists of 2 boron atoms and 6 hydrogen atoms. Out of these 6 hydrogen atoms 4 hydrogen atoms are attached to only one boron atom remaining two hydrogen atoms are attached to both borons through a special type of bond.
-Boron has 3 electrons in valence shell; it cannot complete octet directly through covalent bonds. In the excited state of boron, it has 3 unpaired electrons with 4 orbitals which can involve in bonding. In diborane 2 hydrogen atoms in between two boron atoms are connected to both atoms through a special 3 centered 2 electron bond. In 3 centered 2 electron bonds, 2 electrons are shared in between 3 centers; those are 2 boron atoms and 1 hydrogen atom to satisfy the octet of boron. This type of bond is also considered as regular while calculating hybridization. Since each boron atom has 4 bonds so hybridization will $sp^{ 3 }$ – hybridization.

Therefore, option B is correct.
Note:
has only 3 electrons in the outer shell and can form only 3 hybridized orbitals. So both boron atoms are connected whether it is through direct or indirect bonding.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




