
The $ Trans-3\text{ }methylpent-2-ene $ is:
(A) E
(B) Z
(C) Both (A) and (B)
(D) None of these
Answer
518.4k+ views
Hint: We know that we are given an IUPAC name for the compound. The method of naming organic compounds is known as the IUPAC nomenclature. In the given name, $ suffix\text{ }ene $ is written thus, a double bond must be present.
Complete answer:
We are given an IUPAC name of the compound i.e. $ 3-methylpent-2-ene.\text{ } $ $ pent- $ means that the parent alkane is pentane. Pentane means there are carbon atoms present. $ 3-methyl $ means there is a methyl group attached to the third carbon of the parent alkane. $ pent-2-ene $ means there is a double bond to the second carbon..
Here the molecule has methyl and hydrogen on one side & methyl & ethyl on the other side of the double bond. Now, on the first side higher priority group is methyl & on the other side ethyl. If the two higher priority groups are on the same direction, the molecule is cis, otherwise trans. If two groups of higher priority are on opposite sides of the double bond, the bond is assigned the configuration E.
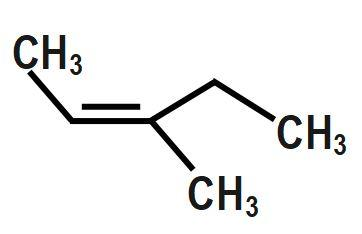
Therefore, the correct answer is option C.
Note:
Remember that don't forget to Assign a number to each substituent according to the carbon atom it is attached to. If there are two substituents on the same carbon, assign the same number to them. The number the carbon atoms in the selected carbon chain from the end which is nearest to the double bond. Count the number of carbon atoms in the chain. This is the parent alkane. The name of alkene is written by replacing suffix ‘ane’ in the parent alkane by ‘ene’ Write the number indicating the position of the double position before prefix ‘ene’.
Complete answer:
We are given an IUPAC name of the compound i.e. $ 3-methylpent-2-ene.\text{ } $ $ pent- $ means that the parent alkane is pentane. Pentane means there are carbon atoms present. $ 3-methyl $ means there is a methyl group attached to the third carbon of the parent alkane. $ pent-2-ene $ means there is a double bond to the second carbon..
Here the molecule has methyl and hydrogen on one side & methyl & ethyl on the other side of the double bond. Now, on the first side higher priority group is methyl & on the other side ethyl. If the two higher priority groups are on the same direction, the molecule is cis, otherwise trans. If two groups of higher priority are on opposite sides of the double bond, the bond is assigned the configuration E.

Therefore, the correct answer is option C.
Note:
Remember that don't forget to Assign a number to each substituent according to the carbon atom it is attached to. If there are two substituents on the same carbon, assign the same number to them. The number the carbon atoms in the selected carbon chain from the end which is nearest to the double bond. Count the number of carbon atoms in the chain. This is the parent alkane. The name of alkene is written by replacing suffix ‘ane’ in the parent alkane by ‘ene’ Write the number indicating the position of the double position before prefix ‘ene’.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




