
: The total number of lone pairs of electrons in melamine is:
A) 4
B) 7
C) 6
D) 9
Answer
567k+ views
Hint: To know the total number of lone pairs of electrons in melamine we must know the structure of melamine. Also, we must know the catenation power and the valency of the elements forming melamine. Knowing the valency helps to determine whether the element will gain or lose electrons to complete its octet.
Complete answer:
A pair of electrons which is not shared with another atom by covalent bond is known as a lone pair. Lone pairs do not participate in bond formation. Lone pairs can be identified by drawing Lewis dot structure.
The molecular formula for melamine is ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{N}}_{\text{6}}}$ or ${{\text{C}}_{\text{3}}}{{\text{N}}_{\text{3}}}{\left( {{\text{N}}{{\text{H}}_{\text{2}}}} \right)_{\text{3}}}$. The IUPAC name of melamine is 1,3,5-triazine.
The structure of melamine is as follows:
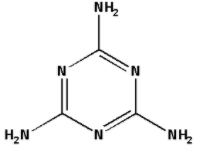
Nitrogen has five electrons in its valence shell. Hydrogen has one electron in its valence shell. Carbon has four electrons in its valence shell. In the structure of melamine, nitrogen bonds with carbon as well as hydrogen. Nitrogen has thus shared three of its valence electrons in bonding. Thus, nitrogen has two electrons in its valence shell which are not shared in bonding. Thus, each nitrogen has one lone pair of electrons in its valence shell.
There are six nitrogen atoms in the structure of melamine and each nitrogen has one lone pair of electrons in its valence shell. Thus, the total number of lone pairs of electrons in melamine is 6.
Thus, the correct answer is option (C) 6.
Note: Melamine has many industrial uses in industries such as glue, dinnerware, flame retardants, etc. Melamine is added to milk to increase its nitrogen content and thus, protein content.
Complete answer:
A pair of electrons which is not shared with another atom by covalent bond is known as a lone pair. Lone pairs do not participate in bond formation. Lone pairs can be identified by drawing Lewis dot structure.
The molecular formula for melamine is ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{N}}_{\text{6}}}$ or ${{\text{C}}_{\text{3}}}{{\text{N}}_{\text{3}}}{\left( {{\text{N}}{{\text{H}}_{\text{2}}}} \right)_{\text{3}}}$. The IUPAC name of melamine is 1,3,5-triazine.
The structure of melamine is as follows:

Nitrogen has five electrons in its valence shell. Hydrogen has one electron in its valence shell. Carbon has four electrons in its valence shell. In the structure of melamine, nitrogen bonds with carbon as well as hydrogen. Nitrogen has thus shared three of its valence electrons in bonding. Thus, nitrogen has two electrons in its valence shell which are not shared in bonding. Thus, each nitrogen has one lone pair of electrons in its valence shell.
There are six nitrogen atoms in the structure of melamine and each nitrogen has one lone pair of electrons in its valence shell. Thus, the total number of lone pairs of electrons in melamine is 6.
Thus, the correct answer is option (C) 6.
Note: Melamine has many industrial uses in industries such as glue, dinnerware, flame retardants, etc. Melamine is added to milk to increase its nitrogen content and thus, protein content.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




