
The total number of electrons in the carbon atom of methyl free radical is:
A) Six
B) Seven
C) Eight
D) Nine
Answer
566.7k+ views
The concepts of valency and the calculation of valence electrons by some of the methods are familiar to us in the lower classes of chemistry.
Now, let us look into what methyl free radical is and the calculation of its valence electrons.
- We have studied the free radicals that occur which are generally defined as any molecule that has the ability to exist independently which possesses unpaired electrons or electrons in an atomic orbital.
- These free radicals are highly unstable and also highly reactive in nature.
- Here, in the above free radical that is methyl free radical is nothing but the carbon bonded to three hydrogens with a presence of one unshared or unpaired electron on it.
Therefore, the structure of methyl free radical can be given as shown below,
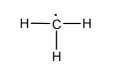
Here since the unpaired electron on carbon is highly reactive and the radical is stable, it reacts quickly to give the molecule required.
We know that one bond is formed by the sharing of one electron each from two different atoms.
Since there are three hydrogen atoms sharing one electron each with carbon we have total electrons $3\times 2=6{{e}^{-}}$ and presence of one unshared electron adds up to give a total of seven electrons.
Thus, the correct answer is option B) Seven.
Note: The calculation of total valence electrons also includes the lone pair of electrons present on an atom and not just the electrons which make a bond and the free radical or unpaired electrons present.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




