
The structure of ${H_2}{O_2}$ is:
A.Planar
B.Non-planar
C.Spherical
D.Linear
Answer
580.8k+ views
Hint: First we should be aware with the term of Hydrogen peroxide which is a compound with peroxy linkage among the molecule. It is a very significant compound with both the oxidising and reducing capabilities. Its structure in not plane due to lone pair repulsion thus possess a non-planar structure.
Complete Step by step answer: Here in the given question statement the question is regarding the compound of Hydrogen peroxide. It is a very important compound in the field of chemistry and is used in multiple occasions as an oxidising as well as reducing agent in both inorganic and organic chemistry. It plays a great role in many processes.
Here are some facts regarding the nature and the molecular structure of ${H_2}{O_2}$ :
Hydrogen peroxide is a compoud which a peroxy linkage made up of oxygen and hydrogen.
The chemical formula of it is ${H_2}{O_2}$ .
In its pure form, hydrogen peroxide is very pale blue and in liquid state. It slightly more viscous than water.
Hydrogen peroxide is one of the simplest derivable and stable peroxide. Peroxide refers to a compound with an oxygen–oxygen single bond.
It is used in multiple tasks but most prominently as an oxidizer, bleaching agent, and antiseptic.
The structure of ${H_2}{O_2}$ is not a normal kind of structure, perhaps a unique one.open book shaped, and thus non-planar.
The structure of ${H_2}{O_2}$ looks like this:
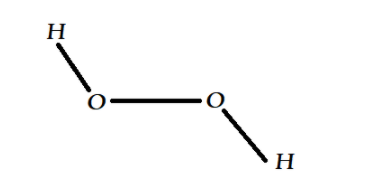
So that provides us the corret option as option B, Non-planar.
Note: Hydrogen peroxide is unstable and slowly decomposes in the presence of light. Because of its instability, hydrogen peroxide is typically stored with a stabilizer in a weakly acidic solution in a dark colored bottle. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases.
Complete Step by step answer: Here in the given question statement the question is regarding the compound of Hydrogen peroxide. It is a very important compound in the field of chemistry and is used in multiple occasions as an oxidising as well as reducing agent in both inorganic and organic chemistry. It plays a great role in many processes.
Here are some facts regarding the nature and the molecular structure of ${H_2}{O_2}$ :
Hydrogen peroxide is a compoud which a peroxy linkage made up of oxygen and hydrogen.
The chemical formula of it is ${H_2}{O_2}$ .
In its pure form, hydrogen peroxide is very pale blue and in liquid state. It slightly more viscous than water.
Hydrogen peroxide is one of the simplest derivable and stable peroxide. Peroxide refers to a compound with an oxygen–oxygen single bond.
It is used in multiple tasks but most prominently as an oxidizer, bleaching agent, and antiseptic.
The structure of ${H_2}{O_2}$ is not a normal kind of structure, perhaps a unique one.open book shaped, and thus non-planar.
The structure of ${H_2}{O_2}$ looks like this:

So that provides us the corret option as option B, Non-planar.
Note: Hydrogen peroxide is unstable and slowly decomposes in the presence of light. Because of its instability, hydrogen peroxide is typically stored with a stabilizer in a weakly acidic solution in a dark colored bottle. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




