
The structural formula of iso-butene is:
A. $C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}$
B. $C{{H}_{3}}CH=CH-C{{H}_{3}}$
C.
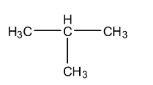
D.
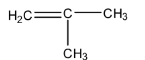


Answer
542.1k+ views
Hint: Structural formula is nothing but the structure of a particular structure of a chemical compound. By using chemical names or IUPAC names only we can draw the structural formula. Structural formulas are also called chemical structure.
Complete step-by-step answer:- In the question it is asked to draw the structure of iso-butene.
- By observing the name we can say that the compound contains four carbon atoms and it is an example of alkene.
- Alkenes have double bonds in their structure.
- The structure of iso-butene is as follows:
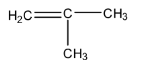
Additional information:
- The IUPAC name of the iso-butene is as follows.
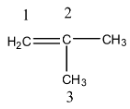
- We have to give the numbering to all the carbon atoms in the organic compound whenever we are going to write the IUPAC name for it.
- At the time of writing IUPAC name we have to give the lowest numbering to the functional groups first.
- Therefore the IUPAC name of the iso-butene is 2-methylprop-1-ene.
- Alkenes are also called as unsaturated compounds due to the presence of double bonds in their structures.
So, the correct option is D.
Note:By using the chemical formula of a compound we cannot find the functional groups those are present in the given compound. By knowing the structure of the compound only we can identify the functional groups present in it. The properties of the organic molecules are going to depend on the functional groups present in it.
Complete step-by-step answer:- In the question it is asked to draw the structure of iso-butene.
- By observing the name we can say that the compound contains four carbon atoms and it is an example of alkene.
- Alkenes have double bonds in their structure.
- The structure of iso-butene is as follows:

Additional information:
- The IUPAC name of the iso-butene is as follows.

- We have to give the numbering to all the carbon atoms in the organic compound whenever we are going to write the IUPAC name for it.
- At the time of writing IUPAC name we have to give the lowest numbering to the functional groups first.
- Therefore the IUPAC name of the iso-butene is 2-methylprop-1-ene.
- Alkenes are also called as unsaturated compounds due to the presence of double bonds in their structures.
So, the correct option is D.
Note:By using the chemical formula of a compound we cannot find the functional groups those are present in the given compound. By knowing the structure of the compound only we can identify the functional groups present in it. The properties of the organic molecules are going to depend on the functional groups present in it.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




