
The state of hybridisation of boron and oxygen atoms in boric acid(${{H}_{3}}B{{O}_{3}}$ ) respectively are:
(a) $s{{p}^{2}}$ and $s{{p}^{2}}$
(b) $s{{p}^{2}}$ and $s{{p}^{3}}$
(c) $s{{p}^{3}}$ and $s{{p}^{2}}$
(d) $s{{p}^{3}}$ and $s{{p}^{3}}$
Answer
593.7k+ views
Hint: Process of combining two or more atomic orbitals from the same atom to form an entirely new different orbital from its components is called hybridisation. During the formation of hydroboric acid, one s and multiple p orbitals of boron and oxygen undergo hybridisation.
Complete answer:
- Boric acid or orthoboric acid has a formula ${{H}_{3}}B{{O}_{3}}$ . The central boron atom forms a bond with three hydroxyl groups. There are three electrons in the outermost valence shell of Boron and all the three can form sigma bonds. There is no lone pair of electrons on Boron; all are engaged in bond formation with the OH- group. Thus it has a trigonal shape and is $s{{p}^{2}}$ hybridised.
- Each oxygen atom forms a sigma bond with hydrogen and boron. The outermost shell of oxygen contains 6 electrons. Out of 2 electrons (one form a sigma bond with boron and other with hydrogen) are engaged in bond formation. Thus, there are 2 lone pairs of electrons on each oxygen atom. So, the oxygen atoms are tetrahedral and for such an atom, the hybridisation is $s{{p}^{3}}$ .
The structure of boric acid is shown as:
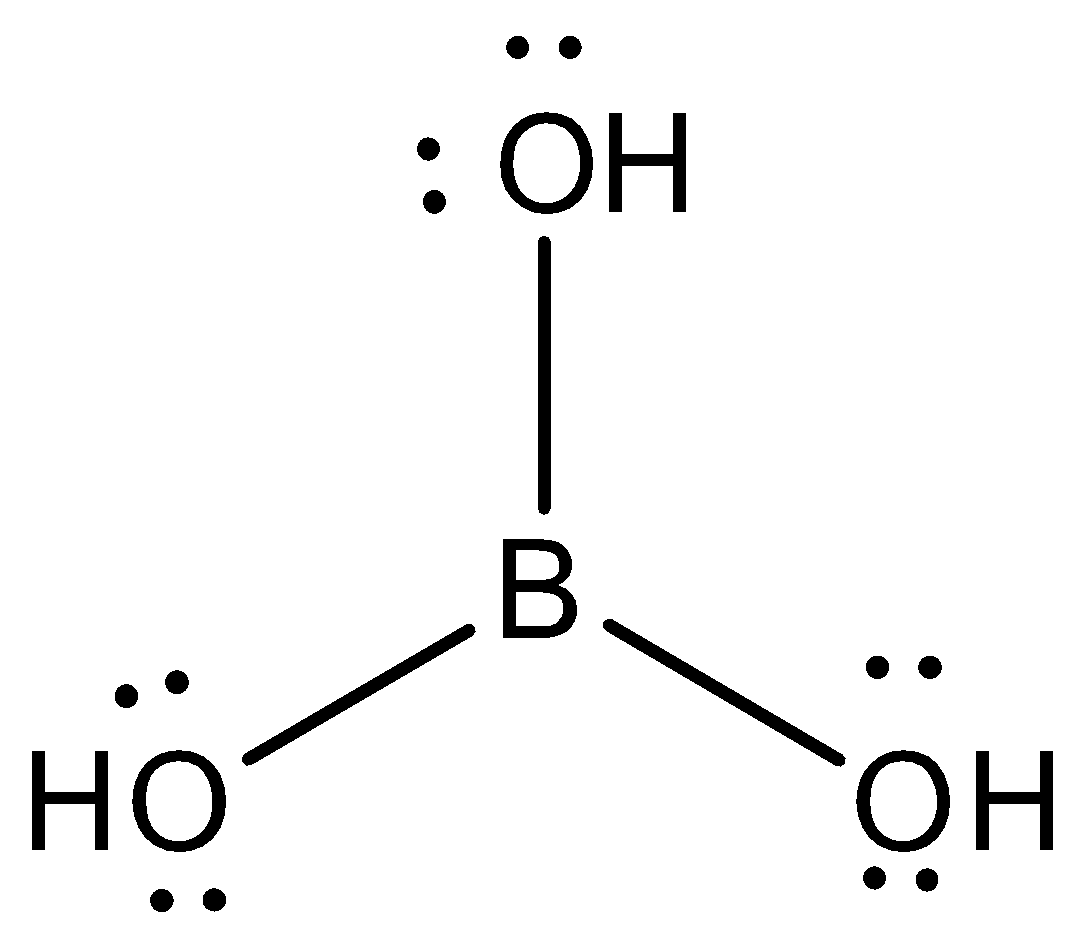
Thus, the state of hybridisation of boron is $s{{p}^{2}}$ and of oxygen is $s{{p}^{3}}$ in hydroboric acid.
So, the correct answer is “Option C”.
Note:
Tetrahedral arrangement in an atom is only seen when the hybridisation of the atom is $s{{p}^{3}}$ . In a trigonal arranged atom, the hybridisation would be $s{{p}^{2}}$ . In hydroboric acid, the boron and the hydroxyl groups all are in the same plane.
Complete answer:
- Boric acid or orthoboric acid has a formula ${{H}_{3}}B{{O}_{3}}$ . The central boron atom forms a bond with three hydroxyl groups. There are three electrons in the outermost valence shell of Boron and all the three can form sigma bonds. There is no lone pair of electrons on Boron; all are engaged in bond formation with the OH- group. Thus it has a trigonal shape and is $s{{p}^{2}}$ hybridised.
- Each oxygen atom forms a sigma bond with hydrogen and boron. The outermost shell of oxygen contains 6 electrons. Out of 2 electrons (one form a sigma bond with boron and other with hydrogen) are engaged in bond formation. Thus, there are 2 lone pairs of electrons on each oxygen atom. So, the oxygen atoms are tetrahedral and for such an atom, the hybridisation is $s{{p}^{3}}$ .
The structure of boric acid is shown as:

Thus, the state of hybridisation of boron is $s{{p}^{2}}$ and of oxygen is $s{{p}^{3}}$ in hydroboric acid.
So, the correct answer is “Option C”.
Note:
Tetrahedral arrangement in an atom is only seen when the hybridisation of the atom is $s{{p}^{3}}$ . In a trigonal arranged atom, the hybridisation would be $s{{p}^{2}}$ . In hydroboric acid, the boron and the hydroxyl groups all are in the same plane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




