
The solubility product of a sparingly soluble salt $AB$ at room temperature is $1.21\times {{10}^{-6}}$. Its molar solubility is:
(A) $1.21\times {{10}^{-6}}$
(B) $1.21\times {{10}^{-3}}$
(C) $1.1\times {{10}^{-4}}$
(D) $1.1\times {{10}^{-3}}$
Answer
374.1k+ views
Hint: When a salt is stirred in water and only a small amount of it gets dissolved but a large amount of it remains undissolved, then the salt is known as sparingly soluble salt. The solubility product of a sparingly soluble salt forming a saturated solution in water is calculated as the product of the concentrations of the ions, raised to a power equal to the number of the ions occurring in the equation representing the dissociation of the electrolyte. The solubility product is denoted by ${{K}_{sp}}$.
Formula Used: The formula for the solubility product depends on the number of ions formed after dissociation of the compound. For a sparingly soluble salt $AB$. The solubility product is given by ${{K}_{sp}}=\left[ {{A}^{+}} \right]\left[ {{B}^{-}} \right]$ where ${{A}^{+}}$ are the cations, ${{B}^{-}}$ are the anions.
Complete Step by Step Solution:
$AB$gets dissociated as
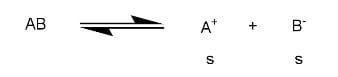
Let solubility be s
${{K}_{sp}}=\left[ {{A}^{+}} \right]\left[ {{B}^{-}} \right]$
${{K}_{sp}}=s\times s$
${{K}_{sp}}={{s}^{2}}$
The given solubility product is ${{K}_{sp}}=1.21\times {{10}^{-6}}$ .
${{s}^{2}}=1.21\times {{10}^{-6}}$
$s=\sqrt{1.21\times {{10}^{-6}}}$
$s=1.1\times {{10}^{-3}}$
Hence, the solubility of $AB$ is $1.1\times {{10}^{-3}}$ moles/litre.
Correct Option: (D) $1.1\times {{10}^{-3}}$
Note: The solubility product depends upon the temperature. It increases with an increase in temperature. This is because with an increase in temperature, solubility increases. Solubility means the tendency of a solute to get dissolved in a solvent to form a solution. The higher the value of a solubility product, the greater the solubility of the substance. It also depends on the common-ion effect; that is, if a common ion is present in the solution, then the solubility product gets lowered.
Formula Used: The formula for the solubility product depends on the number of ions formed after dissociation of the compound. For a sparingly soluble salt $AB$. The solubility product is given by ${{K}_{sp}}=\left[ {{A}^{+}} \right]\left[ {{B}^{-}} \right]$ where ${{A}^{+}}$ are the cations, ${{B}^{-}}$ are the anions.
Complete Step by Step Solution:
$AB$gets dissociated as
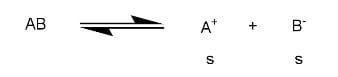
Let solubility be s
${{K}_{sp}}=\left[ {{A}^{+}} \right]\left[ {{B}^{-}} \right]$
${{K}_{sp}}=s\times s$
${{K}_{sp}}={{s}^{2}}$
The given solubility product is ${{K}_{sp}}=1.21\times {{10}^{-6}}$ .
${{s}^{2}}=1.21\times {{10}^{-6}}$
$s=\sqrt{1.21\times {{10}^{-6}}}$
$s=1.1\times {{10}^{-3}}$
Hence, the solubility of $AB$ is $1.1\times {{10}^{-3}}$ moles/litre.
Correct Option: (D) $1.1\times {{10}^{-3}}$
Note: The solubility product depends upon the temperature. It increases with an increase in temperature. This is because with an increase in temperature, solubility increases. Solubility means the tendency of a solute to get dissolved in a solvent to form a solution. The higher the value of a solubility product, the greater the solubility of the substance. It also depends on the common-ion effect; that is, if a common ion is present in the solution, then the solubility product gets lowered.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

What is the median of the first 10 natural numbers class 10 maths CBSE




