
Differentiate between an exothermic and an endothermic reaction.
(A) Endothermic releases heat and exothermic absorbs energy.
(B) Exothermic releases heat and endothermic absorbs energy.
(C) Hydrogen gas is evolved in exothermic but not in endothermic.
(D) Hydrogen gas is evolved in endothermic but not in exothermic.
Answer
571.5k+ views
Hint: There are a number of chemical reactions in chemistry and they are divided into many sub and main reactions. They release energy in the form of sound, light, cold or heat. On the basis of heat two types of reactions are known, one is endothermic and the other is exothermic. Let us discuss these both.
Complete answer:
In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. Since endothermic reactions draw in heat from the surrounding, they tend to cause their environment to cool down. They are also generally non-spontaneous since endothermic reactions field products that are higher in energy than the reactants. As such, the change in enthalpy for an endothermic reaction is always positive.
An exothermic reaction releases energy into the surrounding of the system. In an exothermic reaction, energy is released because the total energy of the products is less than the total energy of the reactions.
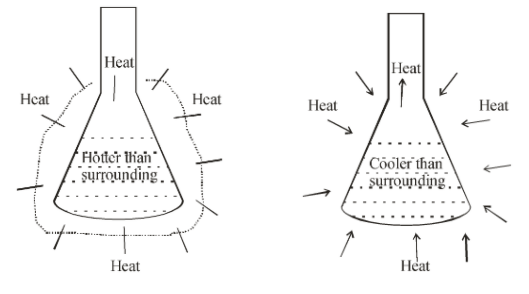
Hence, the correct answer is (B) exothermic reactions heat and endothermic absorb energy.
Note: It should be noted that, Entropy and Enthalpy are distinctive terms, so the change in entropic energy or power can conquer a contrary change in enthalpic energy and make an endothermic reaction ideal.
Complete answer:
In simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. Since endothermic reactions draw in heat from the surrounding, they tend to cause their environment to cool down. They are also generally non-spontaneous since endothermic reactions field products that are higher in energy than the reactants. As such, the change in enthalpy for an endothermic reaction is always positive.
An exothermic reaction releases energy into the surrounding of the system. In an exothermic reaction, energy is released because the total energy of the products is less than the total energy of the reactions.
| ENDOTHERMIC | EXOTHERMIC |
| The reaction which absorbs heat energy from the surrounding is termed as Endothermic reaction. | The reactions which releases heat energy from the surrounding is termed as Exothermic reaction |
| With the progress in such reactions, temperature decreases. | With the progress in such reactions, temperature increases. |
| Energy is supplied to the system. | System releases the energy. |
| The value of change in enthalpy is positive. | The value of change in enthalpy is negative. |
| Enthalpy of products is more than reactant. | Enthalpy of products is less than reactant. |

Hence, the correct answer is (B) exothermic reactions heat and endothermic absorb energy.
Note: It should be noted that, Entropy and Enthalpy are distinctive terms, so the change in entropic energy or power can conquer a contrary change in enthalpic energy and make an endothermic reaction ideal.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




