
The pyrolysis of acetaldehyde under certain conditions can be an intermolecular process. How many alkanes with 2 carbon atoms are formed in the pyrolysis of a mixture of $C{H_3}CHO$ and $C{D_3}CDO$ ?
Answer
582.6k+ views
Hint:Pyrolysis is a thermodynamically process which can be applied to any organic product. It associated thermal treatment. It always produced solid, liquid and non-condensable gases. Pyrolysis process is derived by the treated material composition, temperature of process, residence time and particle size and physical structure. Pyrolysis is a mixture of hydrocarbons, which allows production of fuel and chemical through appropriate refiling methods.
Complete step by step answer:
The pyrolysis of acetaldehyde takes place according to the above image. After pyrolysis of $C{H_3}CHO$ and $C{D_3}CDO$ which produces two moles of alkanes with 2 carbons.
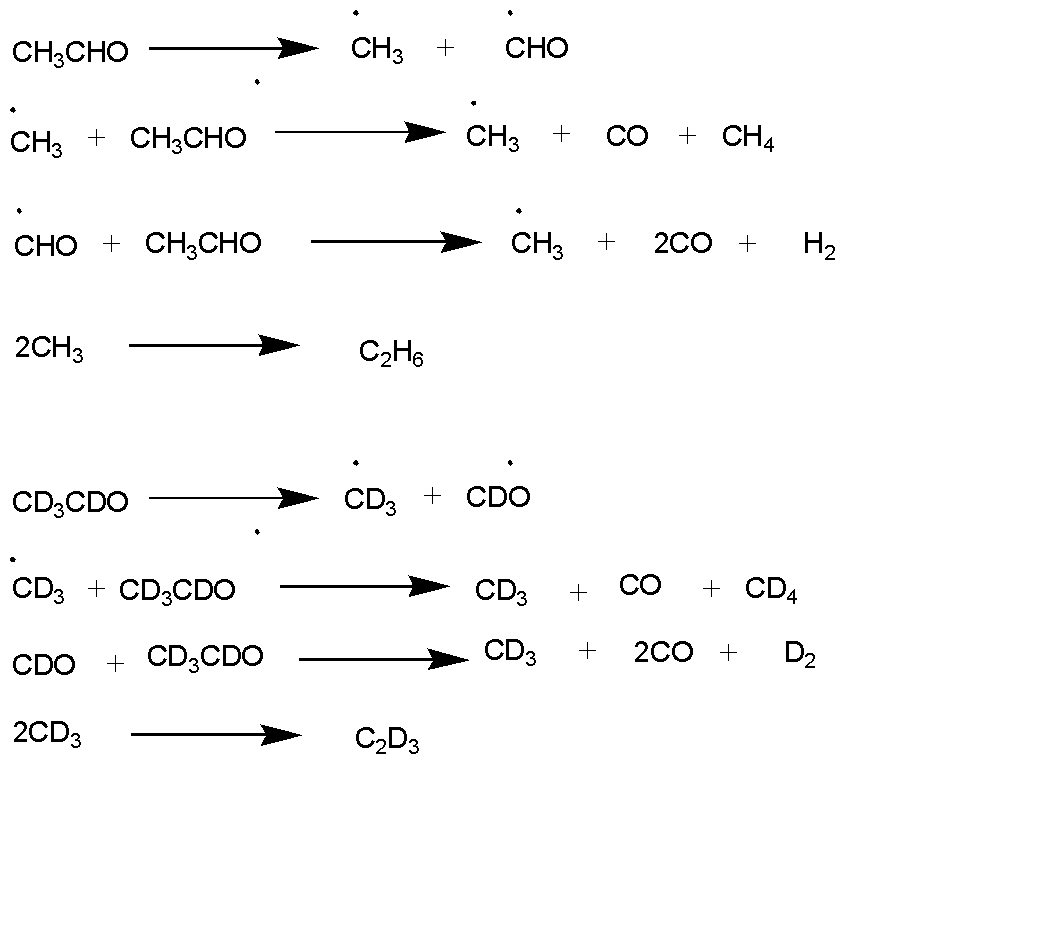
This reaction shows that pyrolysis reaction occurs in hydrocarbons which produce alkenes with 2 carbons. Acetaldehyde is the primary molecule that yields methyl and formyl radicals, and the subsequent to the formation of hydrogen. The reaction formation of free radicals shows the reaction of pyrolysis.
Additional information:
Pyrolysis is the process which takes place to the imagination. Acetaldehyde, produced from the ethanol. Acetaldehyde deposited in the nasal cavity and upper respiratory tract. It in the nasal cavity is influenced by its solubility and inspiratory flow rate.
Note:Pyrolysis is thermodynamically processed. This process contains organic compounds. This is derived by the free radical reaction and it forms a product which contains 2 atoms of carbon. This process occurs in the hydrocarbons which form alkene as a product and subsequent to the formation of hydrogen.
Complete step by step answer:
The pyrolysis of acetaldehyde takes place according to the above image. After pyrolysis of $C{H_3}CHO$ and $C{D_3}CDO$ which produces two moles of alkanes with 2 carbons.

This reaction shows that pyrolysis reaction occurs in hydrocarbons which produce alkenes with 2 carbons. Acetaldehyde is the primary molecule that yields methyl and formyl radicals, and the subsequent to the formation of hydrogen. The reaction formation of free radicals shows the reaction of pyrolysis.
Additional information:
Pyrolysis is the process which takes place to the imagination. Acetaldehyde, produced from the ethanol. Acetaldehyde deposited in the nasal cavity and upper respiratory tract. It in the nasal cavity is influenced by its solubility and inspiratory flow rate.
Note:Pyrolysis is thermodynamically processed. This process contains organic compounds. This is derived by the free radical reaction and it forms a product which contains 2 atoms of carbon. This process occurs in the hydrocarbons which form alkene as a product and subsequent to the formation of hydrogen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




