
The product (C) and (D) are:
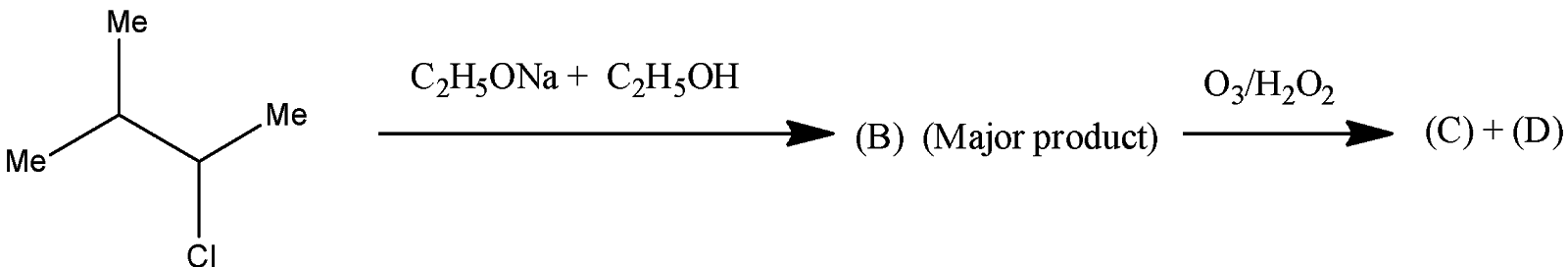
A. Acetone+Ethanal
B. Acetone+Ethanoic acid
C. Isobutanal+Methanal
D. Isobutanoic acid+Methanoic acid

Answer
583.2k+ views
Hint: We know that alkyl chlorine is an organic compound in which one hydrogen atom of alkane is replaced by a halogen (fluorine, chlorine) etc. An organic reaction in which removal of two substituents occurs in one or two steps is termed as elimination reaction.
Complete step by step answer: Let’s discuss the Saytzeff rule first. According to this rule, the major product in the elimination reaction is the one which contains less number of hydrogen atoms on the double bonded carbon atom.
Now, come to the question.
The reactant undergoes elimination reaction
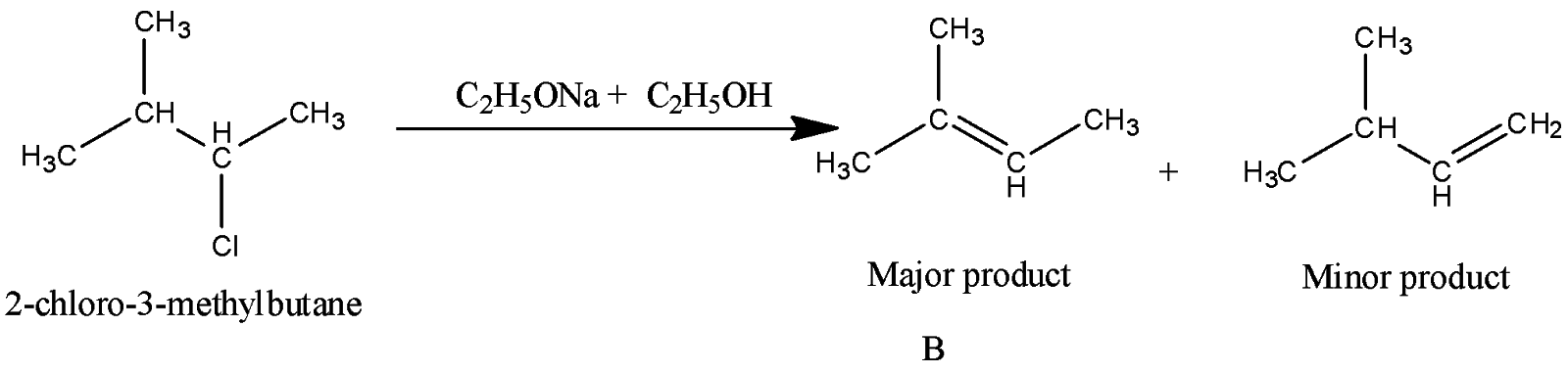
The major product is as it has less number of hydrogen atoms on the double bonded carbon atom. (Saytzeff rule)
Now, B reacts with ${{\rm{O}}_{\rm{3}}}$. We know that the reaction of alkene with ozone forms an ozonide, followed by hydrolysis of ozonide to produce aldehyde or ketone. The ozonolysis of B produces acetone and ethanal. ${{\rm{H}}_{\rm{2}}}{{\rm{O}}_{\rm{2}}}$ is an oxidizing agent.
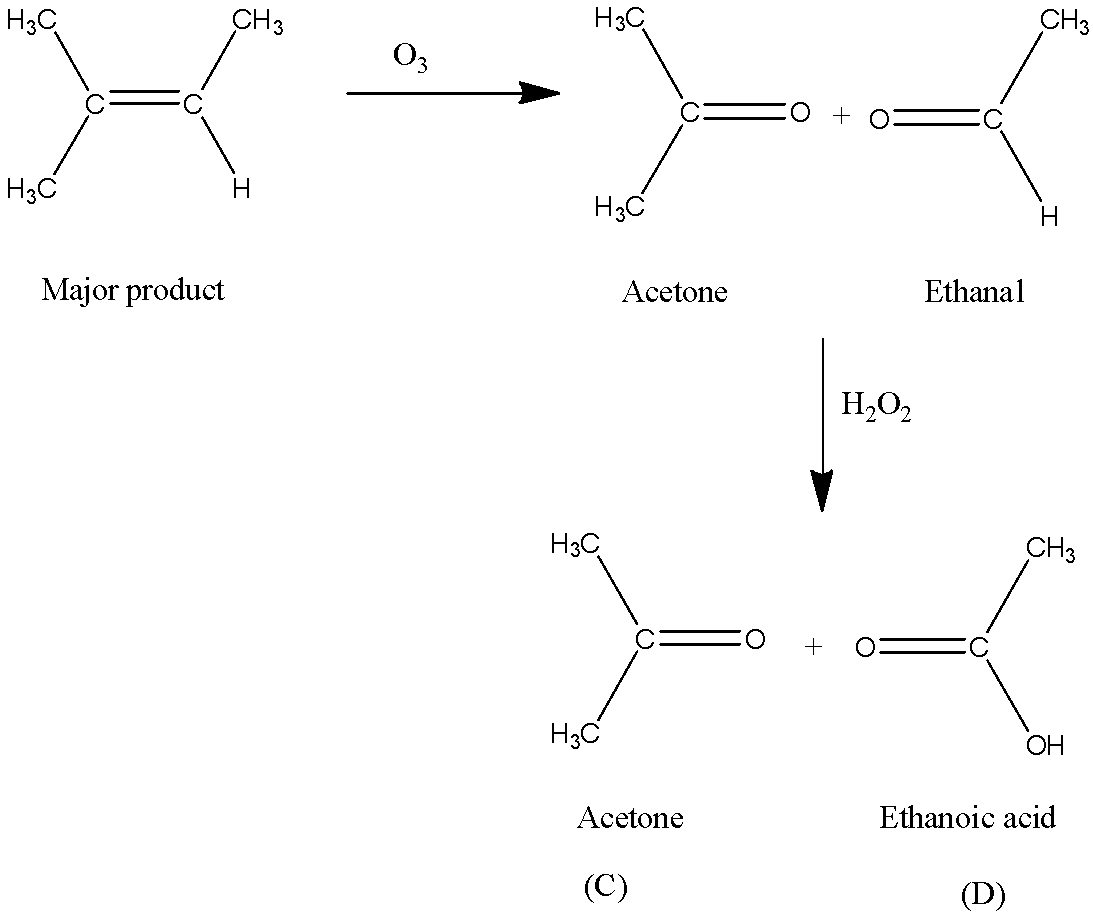
As acetone has no particular hydrogen atom, it does not undergo oxidation reaction. But ethanol is oxidized by hydrogen peroxide $\left( {{{\rm{H}}_{\rm{2}}}{{\rm{O}}_{\rm{2}}}} \right)$.
Now, we write the complete reaction.
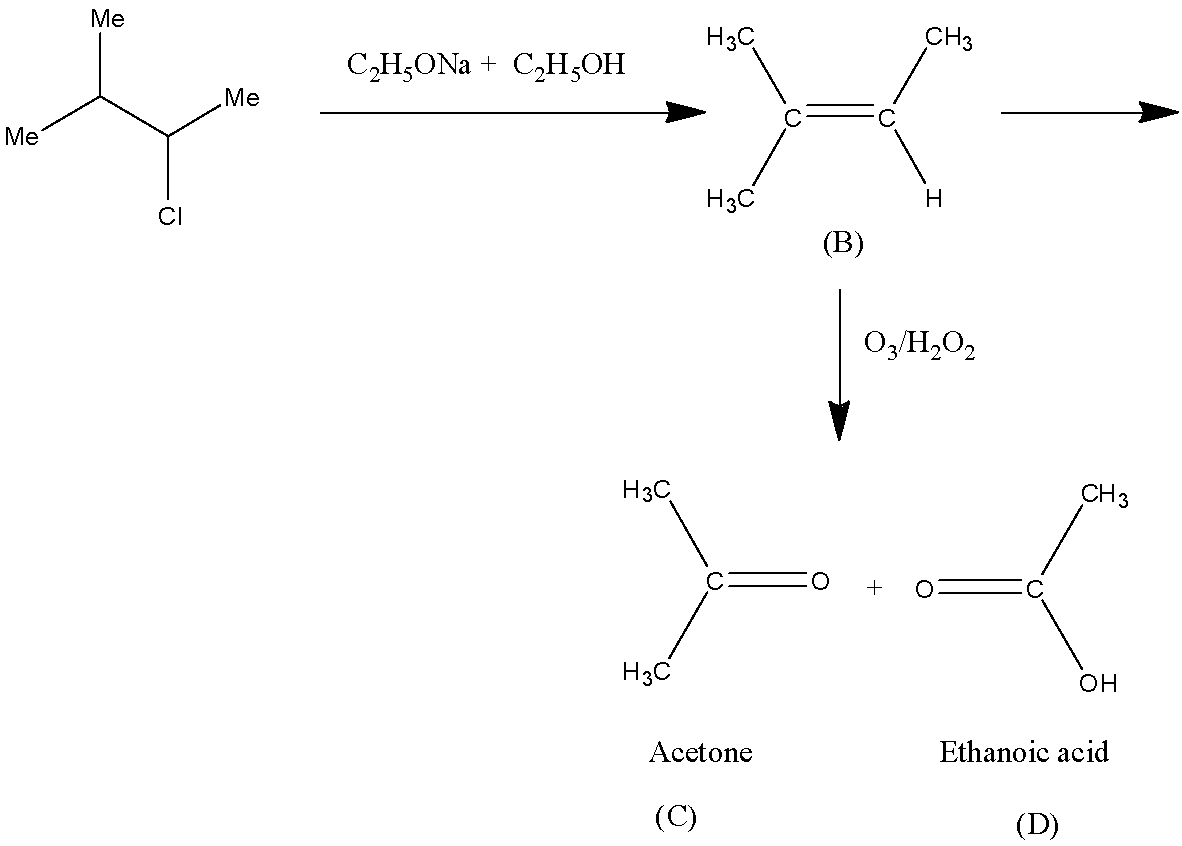
Therefore, (C) is acetone and (D) is ethanoic acid. Hence, option B is the correct option.
Note: Remember that Alkyl halide undergoes two types of reactions in presence of base, that is, substitution reaction and elimination reaction. In substitution reaction, halide atom is substituted by alcohol group and in elimination reaction halide atom is removed and double bond forms, that means, elimination reaction gives an alkene and the product of substitution reaction is an alcohol.
Complete step by step answer: Let’s discuss the Saytzeff rule first. According to this rule, the major product in the elimination reaction is the one which contains less number of hydrogen atoms on the double bonded carbon atom.
Now, come to the question.
The reactant undergoes elimination reaction

The major product is as it has less number of hydrogen atoms on the double bonded carbon atom. (Saytzeff rule)
Now, B reacts with ${{\rm{O}}_{\rm{3}}}$. We know that the reaction of alkene with ozone forms an ozonide, followed by hydrolysis of ozonide to produce aldehyde or ketone. The ozonolysis of B produces acetone and ethanal. ${{\rm{H}}_{\rm{2}}}{{\rm{O}}_{\rm{2}}}$ is an oxidizing agent.

As acetone has no particular hydrogen atom, it does not undergo oxidation reaction. But ethanol is oxidized by hydrogen peroxide $\left( {{{\rm{H}}_{\rm{2}}}{{\rm{O}}_{\rm{2}}}} \right)$.
Now, we write the complete reaction.

Therefore, (C) is acetone and (D) is ethanoic acid. Hence, option B is the correct option.
Note: Remember that Alkyl halide undergoes two types of reactions in presence of base, that is, substitution reaction and elimination reaction. In substitution reaction, halide atom is substituted by alcohol group and in elimination reaction halide atom is removed and double bond forms, that means, elimination reaction gives an alkene and the product of substitution reaction is an alcohol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




