
The pressure-volume work for an ideal gas can be calculated by using the expression $\text{ w = }-\int\limits_{\text{V}}^{{{\text{V}}_{\text{1}}}}{{{p}_{ex}}\text{dV}}\text{ }$ . The work can also be calculated from the $\text{ pV }$plot by using the area under the curve within the specified limits. When an ideal gas is compressed (a) reversibly or (b) irreversibly from volume $\text{ }{{\text{V}}_{\text{1}}}\text{ }$ to $\text{ }{{\text{V}}_{\text{f}}}\text{ }$. Choose the correct option.
A) $\text{ w}\left( \text{reversible} \right)\text{ = w}\left( \text{irreversible} \right)\text{ }$
B) $\text{ w(reversible) }<\text{ w(irreversible) }$
C) \[\text{ w}\left( \text{reversible} \right)\text{ = }-\text{w}\left( \text{irreversible} \right)\text{ }\]
D) \[\text{ w}\left( \text{reversible} \right)\text{ = w}\left( \text{irreversible} \right)\text{ + }{{\text{p}}_{\text{ex}}}\Delta \text{V }\]
Answer
582.6k+ views
Hint: The reversible process is a process carried out slowly thus the energy required for the process is very small. However, the irreversible reaction is a sudden process. Thus the process utilizes a larger amount of energy. The work done can be depicted by the $\text{ PV }$ diagram.
Complete step by step answer:
The reversible reaction is a process carried out slowly so that the driving force is infinitesimally greater than the opposing force. That is, during the reversible reaction the change in the parameter of the system takes place slowly. Such that the energy lost during the work done by the system is very small.
On the other hand, the irreversible reactions are the process that does not take place infinitesimally slowly, instead, these are rapid and sudden reactions. The system changes from the initial stage to the final stage without any intermediate stage. Thus, the energy dissipated during the change in the system is greater.
Consider a gas undergoes the isothermal compression from the volume $\text{ }{{\text{V}}_{\text{1}}}\text{ }$ to the $\text{ }{{\text{V}}_{2}}\text{ }$. The external pressure is higher than the P, the pressure of the system. Let the external pressure apply to the be \[\text{ }{{\text{P}}_{\text{rev}}}\text{ }\]. During the compression of the gas, the pressure of the system increases, and the volume of the system decreases. These parameters have opposite signs as before.
For reversible reaction, the P-V curve is as shown below,
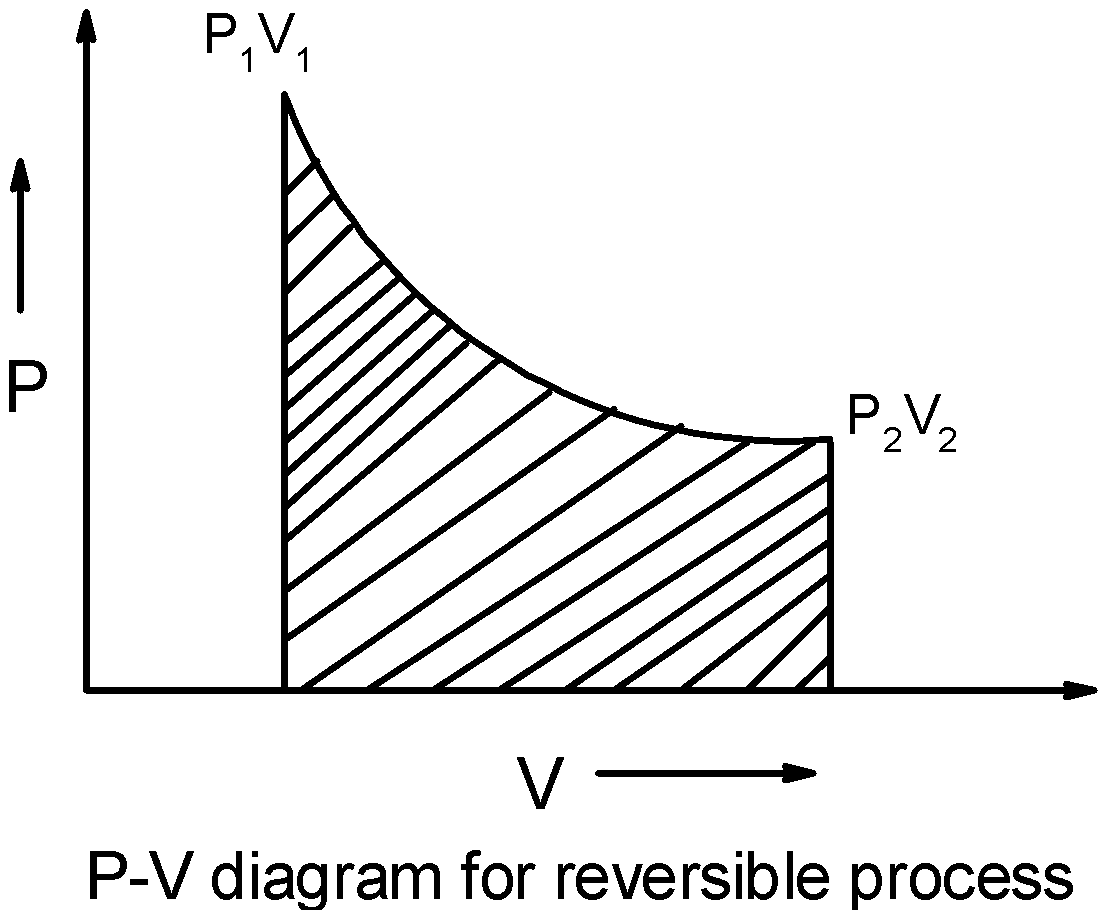
The energy utilized or dissipated is small, thus the p-V curve is deep.
But the irreversible reaction is associated with the sudden change of the system the $\text{ }{{\text{P}}_{\text{1}}}{{\text{V}}_{\text{1}}}\text{ }\to \text{ }{{\text{P}}_{\text{2}}}{{\text{V}}_{\text{2}}}\text{ }$ thus, the curve is a straight line joining the initial and final state of the system.
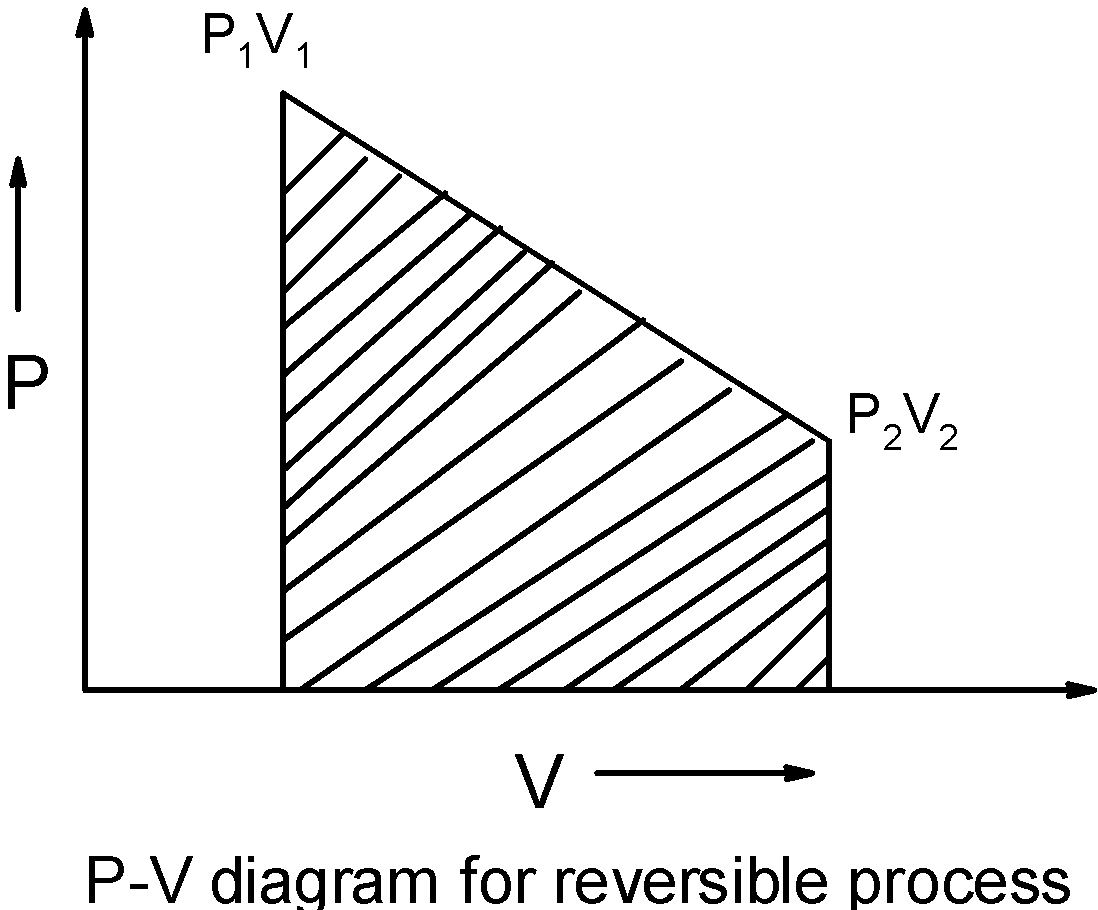
If we calculate the area under the curve, we can see that the area under the curve for the reversible reaction is less than the area under the curve formed by the isotherm irreversible compression of the gas.
Thus, we have $\text{ w(reversible) }<\text{ w(irreversible) }$
So, the correct answer is “Option B”.
Note: Note that, the magnitude of the work done by the system depends on the magnitude of the opposing (external) pressure. The closer is the opposing pressure to the pressure of the gas, the greater is the work done. Thus, the condition for the maximum work done coincides with the thermodynamic reversibility.
Complete step by step answer:
The reversible reaction is a process carried out slowly so that the driving force is infinitesimally greater than the opposing force. That is, during the reversible reaction the change in the parameter of the system takes place slowly. Such that the energy lost during the work done by the system is very small.
On the other hand, the irreversible reactions are the process that does not take place infinitesimally slowly, instead, these are rapid and sudden reactions. The system changes from the initial stage to the final stage without any intermediate stage. Thus, the energy dissipated during the change in the system is greater.
Consider a gas undergoes the isothermal compression from the volume $\text{ }{{\text{V}}_{\text{1}}}\text{ }$ to the $\text{ }{{\text{V}}_{2}}\text{ }$. The external pressure is higher than the P, the pressure of the system. Let the external pressure apply to the be \[\text{ }{{\text{P}}_{\text{rev}}}\text{ }\]. During the compression of the gas, the pressure of the system increases, and the volume of the system decreases. These parameters have opposite signs as before.
For reversible reaction, the P-V curve is as shown below,

The energy utilized or dissipated is small, thus the p-V curve is deep.
But the irreversible reaction is associated with the sudden change of the system the $\text{ }{{\text{P}}_{\text{1}}}{{\text{V}}_{\text{1}}}\text{ }\to \text{ }{{\text{P}}_{\text{2}}}{{\text{V}}_{\text{2}}}\text{ }$ thus, the curve is a straight line joining the initial and final state of the system.

If we calculate the area under the curve, we can see that the area under the curve for the reversible reaction is less than the area under the curve formed by the isotherm irreversible compression of the gas.
Thus, we have $\text{ w(reversible) }<\text{ w(irreversible) }$
So, the correct answer is “Option B”.
Note: Note that, the magnitude of the work done by the system depends on the magnitude of the opposing (external) pressure. The closer is the opposing pressure to the pressure of the gas, the greater is the work done. Thus, the condition for the maximum work done coincides with the thermodynamic reversibility.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




