
What will be the prefix in the given compound?
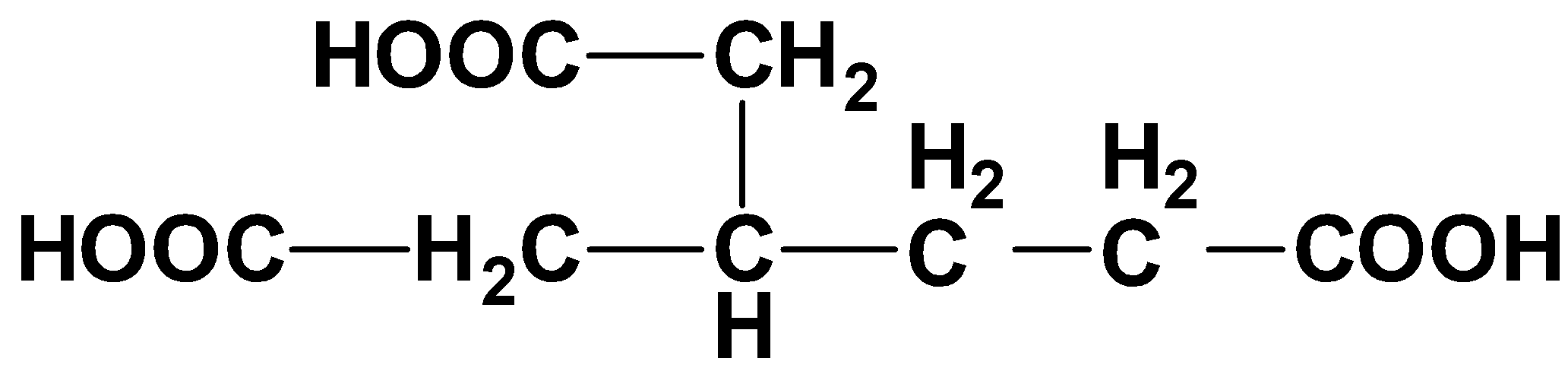
A. Methyl Carboxy
B. Carboxymethyl
C. Carboxyethyl
D. Ethyl Carboxy

Answer
554.1k+ views
Hint: If we look at the structure of the compound, it has three carboxylic acid groups at the three ends. We will start naming the compound from the left-most carbon atom and this way we will find a \[C{H_2} - {C_2}{H_5}\] \[C{H_2} - COOH\] group as a side branch.
Complete step by step answer:
We know that a prefix is a word, letter or number which is placed in the starting or the initial introduction of any word. The given compound is a branched-chain compound with three carboxylic acid groups. To solve this question, we will look at each of the four options one by one.
The first prefix is methyl carboxy, whenever we name a carboxylic acid, we never use the word methyl or ethyl (suggesting the number of carbon) before the word carboxy (suggesting the presence of carboxylic acid). So, this option is incorrect.
The second prefix is carboxymethyl. If we look at the compound it has a branched chain of carboxy methyl group i.e., \[C{H_2} - COOH\]. So, we use the prefix as carboxy methyl. As mentioned above, carboxy suggests the \[COOH\] group and methyl represents the \[C{H_2}\] group.
The third option is carboxy ethyl. This cannot be the correct option because it is very clear from the structure of the compound that there is \[C{H_2}\] group (methyl group) instead of \[{C_2}{H_5}\] group (ethyl group).
The fourth option is ethyl carboxy. This also cannot be the correct option because firstly, the compound has carboxy methyl group and secondly, the word ethyl cannot come before the word carboxy while naming any acid.
So, the correct answer is Option B.
Note: In this compound, there are three ways from where we can start numbering the carbon atoms, but we always choose that chain which includes the maximum number of carbon atoms and starting with a functional group.
Complete step by step answer:
We know that a prefix is a word, letter or number which is placed in the starting or the initial introduction of any word. The given compound is a branched-chain compound with three carboxylic acid groups. To solve this question, we will look at each of the four options one by one.
The first prefix is methyl carboxy, whenever we name a carboxylic acid, we never use the word methyl or ethyl (suggesting the number of carbon) before the word carboxy (suggesting the presence of carboxylic acid). So, this option is incorrect.
The second prefix is carboxymethyl. If we look at the compound it has a branched chain of carboxy methyl group i.e., \[C{H_2} - COOH\]. So, we use the prefix as carboxy methyl. As mentioned above, carboxy suggests the \[COOH\] group and methyl represents the \[C{H_2}\] group.
The third option is carboxy ethyl. This cannot be the correct option because it is very clear from the structure of the compound that there is \[C{H_2}\] group (methyl group) instead of \[{C_2}{H_5}\] group (ethyl group).
The fourth option is ethyl carboxy. This also cannot be the correct option because firstly, the compound has carboxy methyl group and secondly, the word ethyl cannot come before the word carboxy while naming any acid.
So, the correct answer is Option B.
Note: In this compound, there are three ways from where we can start numbering the carbon atoms, but we always choose that chain which includes the maximum number of carbon atoms and starting with a functional group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




