
The polymer used in the manufacture of 'orlon' is:
A. PTFE
B. PAN
C. PMMA
D. PVC
Answer
595.5k+ views
Hint: Orlon is a type of a synthetic acrylic fiber, and has a major use in the textile industry. The fiber is very suitable for outdoor usage as it is very resistant to the sunlight and atmospheric gases. And it does not shrink easily and has a soft and warm feel.
Complete step by step solution:
As we know about what orlon is let’s proceed towards its polymerization reaction. Orlon is prepared by the addition reaction of vinyl cyanide or in other words acrylonitrile molecules or can be called as PAN which stands for Poly acrylonitrile. In this reaction the double bonds of the central carbons are broken to form single bonds and an additional new bond is formed with a new Carbon.
And hence Correct option is B
The reaction is as follows:
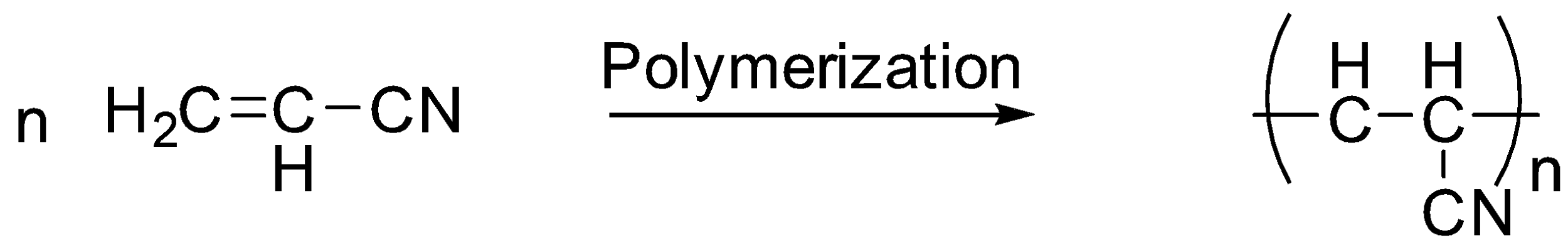
Additional Information: As orlon is an acrylic fiber let us know what these acrylic fibers are they can be defined as synthetic fibers which are made from a polymer known as PAN or polyacrylonitrile which has a molecular weight of around \[100,000\] , and is made up of about $1900$ monomer units. For a fiber to be called an "acrylic", the polymer should contain at least $85\% $ of the acrylonitrile monomer. For example: vinyl acetate or methyl acrylate. The first acrylic fiber was created by DuPont and trademarked it with the name of Orlon.
Note: Acrylic fibers have another modified subclass of fiber known as the Modacrylic fiber and it consists of at least $35\% $ and at most $85\% $ acrylonitrile monomer. Some of the common monomer examples are: vinyl chloride, vinylidene chloride and vinyl bromide
Complete step by step solution:
As we know about what orlon is let’s proceed towards its polymerization reaction. Orlon is prepared by the addition reaction of vinyl cyanide or in other words acrylonitrile molecules or can be called as PAN which stands for Poly acrylonitrile. In this reaction the double bonds of the central carbons are broken to form single bonds and an additional new bond is formed with a new Carbon.
And hence Correct option is B
The reaction is as follows:

Additional Information: As orlon is an acrylic fiber let us know what these acrylic fibers are they can be defined as synthetic fibers which are made from a polymer known as PAN or polyacrylonitrile which has a molecular weight of around \[100,000\] , and is made up of about $1900$ monomer units. For a fiber to be called an "acrylic", the polymer should contain at least $85\% $ of the acrylonitrile monomer. For example: vinyl acetate or methyl acrylate. The first acrylic fiber was created by DuPont and trademarked it with the name of Orlon.
Note: Acrylic fibers have another modified subclass of fiber known as the Modacrylic fiber and it consists of at least $35\% $ and at most $85\% $ acrylonitrile monomer. Some of the common monomer examples are: vinyl chloride, vinylidene chloride and vinyl bromide
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




