
The peptide that gives positive ceric ammonium nitrate and carbylamine test is:
A.Lys-Asp
B.Ser-Lys
C.Gln-Asp
D.Asp-Gln
Answer
591.9k+ views
Hint: Peptides are basically a small or short chain with anywhere between two to fifty amino acids. These small chains are held together by peptide bonds, hence the name.
Complete step by step answer:
The Carbylamine test is basically a method for detecting or determining primary amines given in the present compounds, by heating up the given substance with chloroform in a basic solution. The test shows positive results for the presence of a primary amine, by emitting a characteristic foul smell, which is caused by the release of isocyanide. The general carbylamine reaction can be represented as follows:
\[R - N{H_2} + CHC{l_3} + 3KOH \to RNC + 3KCl + 3{H_2}O\]
Ceric ammonium nitrate test is used for identifying the existence of alcoholic functional groups. In this test, a sample of the given compound is dissolved in an appropriate solvent. If the solution turns red, then the test result is positive.
The given peptides are:
Lysine (Lys)-
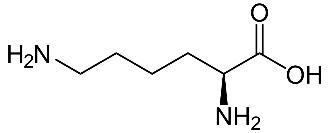
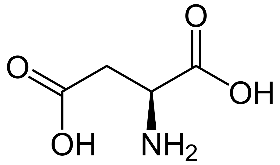
Aspartic acid (Asp)-
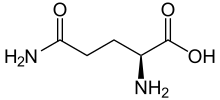
Glutamine (Gln) –
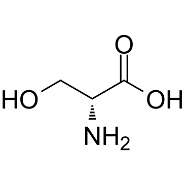
Serine (Ser) –
We can see that as a combination, only serine and lysine would be able to give a positive result for both the tests due to the presence of -OH and - \[N{H_2}\] functional groups.
Hence, Option B is the correct option.
Note:
A peptide bond is formed when the carboxyl group of one molecule reacts with the amino group of another molecule releasing a molecule of water.
Complete step by step answer:
The Carbylamine test is basically a method for detecting or determining primary amines given in the present compounds, by heating up the given substance with chloroform in a basic solution. The test shows positive results for the presence of a primary amine, by emitting a characteristic foul smell, which is caused by the release of isocyanide. The general carbylamine reaction can be represented as follows:
\[R - N{H_2} + CHC{l_3} + 3KOH \to RNC + 3KCl + 3{H_2}O\]
Ceric ammonium nitrate test is used for identifying the existence of alcoholic functional groups. In this test, a sample of the given compound is dissolved in an appropriate solvent. If the solution turns red, then the test result is positive.
The given peptides are:
Lysine (Lys)-

Aspartic acid (Asp)-

Glutamine (Gln) –

Serine (Ser) –

We can see that as a combination, only serine and lysine would be able to give a positive result for both the tests due to the presence of -OH and - \[N{H_2}\] functional groups.
Hence, Option B is the correct option.
Note:
A peptide bond is formed when the carboxyl group of one molecule reacts with the amino group of another molecule releasing a molecule of water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




