
The number of equivalent \[Cl - O\] bonds in $C{l_2}{O_7}$ is:
A. $6$
B. $4$
C. $8$
D. $2$
Answer
589.2k+ views
Hint: A chemical substance composed from the atoms of different elements which are bonded together by chemical bonds is known as chemical compound. Dichlorine heptoxide is a chemical compound, chlorine and oxygen elements have contributed to this compound.
Complete step by step answer:
A chemical substance composed of many molecules made up of atoms of more than one element which are held together by chemical bonds is known as a chemical compound. If it is made up of a single type of element then it is not called as a compound rather it is a molecule.
Dichlorine heptoxide is a chemical compound composed of oxygen and chlorine element atoms. The chemical formula of dichlorine heptoxide is $C{l_2}{O_7}$. Dichlorine heptoxide is basically the anhydride of perchloric acid. When maximum possible water molecules are removed from the perchloric acid, dichlorine heptoxide is produced. The dehydrating agent used in this distillation process is phosphorus pentoxide.
Structure of dichlorine heptoxide ($C{l_2}{O_7}$) is:
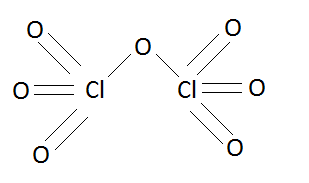
In the above structure we can see that one oxygen atom is shared by both of the chlorine atoms and rest oxygen atoms are distributed equally. This sharing of oxygen atoms or the linkage between three atoms is known as peroxy linkage.
So in this structure dichlorine heptoxide has two chlorine oxygen bonds (\[Cl - O\]) which are situated in the middle of this chemical substance.
Hence the number of equivalent \[Cl - O\] bonds in $C{l_2}{O_7}$ are two.
So, the correct answer is Option D .
Note:
Basically dichlorine heptaoxide is an unstable compound or we can say it is an endergonic material which means intrinsic unstable. Easily it decomposes into its constituent elements with release of energy.
Complete step by step answer:
A chemical substance composed of many molecules made up of atoms of more than one element which are held together by chemical bonds is known as a chemical compound. If it is made up of a single type of element then it is not called as a compound rather it is a molecule.
Dichlorine heptoxide is a chemical compound composed of oxygen and chlorine element atoms. The chemical formula of dichlorine heptoxide is $C{l_2}{O_7}$. Dichlorine heptoxide is basically the anhydride of perchloric acid. When maximum possible water molecules are removed from the perchloric acid, dichlorine heptoxide is produced. The dehydrating agent used in this distillation process is phosphorus pentoxide.
Structure of dichlorine heptoxide ($C{l_2}{O_7}$) is:

In the above structure we can see that one oxygen atom is shared by both of the chlorine atoms and rest oxygen atoms are distributed equally. This sharing of oxygen atoms or the linkage between three atoms is known as peroxy linkage.
So in this structure dichlorine heptoxide has two chlorine oxygen bonds (\[Cl - O\]) which are situated in the middle of this chemical substance.
Hence the number of equivalent \[Cl - O\] bonds in $C{l_2}{O_7}$ are two.
So, the correct answer is Option D .
Note:
Basically dichlorine heptaoxide is an unstable compound or we can say it is an endergonic material which means intrinsic unstable. Easily it decomposes into its constituent elements with release of energy.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Explain zero factorial class 11 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

State and prove Bernoullis theorem class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




