
The most electronegative element of period-3 is:
A. $\text{Ar}$
B. $\text{S}$
C. $\text{Si}$
D. $\text{Cl}$
Answer
593.1k+ views
Hint:Electronegativity is affected by atomic number of elements and the distance at which its valence electrons lie from the nucleus. As we move from left to right in the periodic table, the electronegativity of elements increases and electropositivity of elements decreases.
Complete step by step answer:
Electronegativity is the measure of tendency of an atom to attract a bonding pair of electrons. It is represented by the symbol $'\chi '$. Let us now see the trends in periodic table of electronegativity:
Electronegativity increases from left to right along a period and decreases on descending down the group. This is because electronegativity is determined by factors like the nuclear charge and location of other electrons in the shells (the farther the valence electrons from the nucleus will be it will experience the less positive charge both because of the increased distance from the nucleus and electrons in the lower energy orbitals will shield the valence electrons from the nucleus).
Thus, fluorine is the most electronegative of the elements (ignoring noble gases) and caesium is the least electronegative.
After, seeing the general trends in electronegativity, we find the correct answer easily. First talk about element $\text{Ar}$ (argon) is noble gas present at the end of period 3 with configuration $\left( \text{1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{6}}\text{3}{{\text{s}}^{2}}\text{3}{{\text{p}}^{6}} \right)$. Noble gases have eight electrons in their outer shells, so they don’t want to attract any more electrons for stability. Electronegativity measures the attraction between an atom and an incoming electron; noble gases do not have electronegativity. Argon is least electronegative.
- Chlorine has $\left( \text{1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{6}}\text{3}{{\text{s}}^{2}}\text{3}{{\text{p}}^{5}} \right)$ configuration. Placed at the end of the periodic table before argon. It follows general trends of periodic table. So, it is the most electronegative element in period 3.
- Sulphur has $\left( \text{1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{6}}\text{3}{{\text{s}}^{2}}\text{3}{{\text{p}}^{4}} \right)$ configuration. In the periodic table, it is placed before chlorine. It follows general trends of periodic table. Less electronegative element after chlorine in period 3.
- Silicon has $\left( \text{1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{6}}\text{3}{{\text{s}}^{2}}\text{3}{{\text{p}}^{2}} \right)$ configuration. In the periodic table, it is placed before phosphorus. It follows general trends of periodic table. It has lower electronegativity than chlorine and sulphur in period 3.
The correct order of electronegativity is $\text{Cl}>\text{S}>\text{Si}>\text{Ar}$.
The correct answer to this question is option D.
Note:
Just by seeing the general trend that electronegativity increases from left to right, do not mark ‘$\text{Ar}$’ as the most electronegative element (being placed at the end of that period). Please take a note that $\text{Ar}$ is noble gas whose electronegativity is not counted.
Trend of electronegativity is as follows-
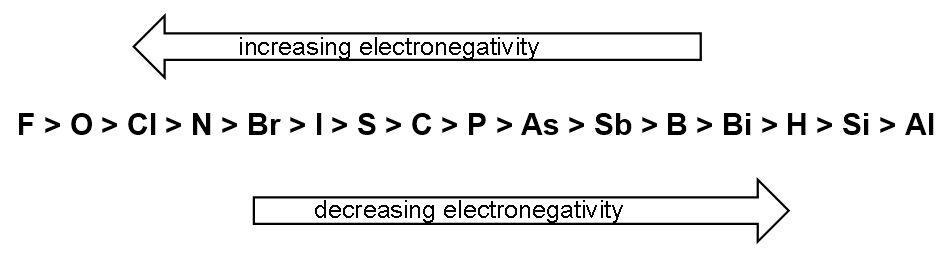
Complete step by step answer:
Electronegativity is the measure of tendency of an atom to attract a bonding pair of electrons. It is represented by the symbol $'\chi '$. Let us now see the trends in periodic table of electronegativity:
Electronegativity increases from left to right along a period and decreases on descending down the group. This is because electronegativity is determined by factors like the nuclear charge and location of other electrons in the shells (the farther the valence electrons from the nucleus will be it will experience the less positive charge both because of the increased distance from the nucleus and electrons in the lower energy orbitals will shield the valence electrons from the nucleus).
Thus, fluorine is the most electronegative of the elements (ignoring noble gases) and caesium is the least electronegative.
After, seeing the general trends in electronegativity, we find the correct answer easily. First talk about element $\text{Ar}$ (argon) is noble gas present at the end of period 3 with configuration $\left( \text{1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{6}}\text{3}{{\text{s}}^{2}}\text{3}{{\text{p}}^{6}} \right)$. Noble gases have eight electrons in their outer shells, so they don’t want to attract any more electrons for stability. Electronegativity measures the attraction between an atom and an incoming electron; noble gases do not have electronegativity. Argon is least electronegative.
- Chlorine has $\left( \text{1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{6}}\text{3}{{\text{s}}^{2}}\text{3}{{\text{p}}^{5}} \right)$ configuration. Placed at the end of the periodic table before argon. It follows general trends of periodic table. So, it is the most electronegative element in period 3.
- Sulphur has $\left( \text{1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{6}}\text{3}{{\text{s}}^{2}}\text{3}{{\text{p}}^{4}} \right)$ configuration. In the periodic table, it is placed before chlorine. It follows general trends of periodic table. Less electronegative element after chlorine in period 3.
- Silicon has $\left( \text{1}{{\text{s}}^{2}}\text{2}{{\text{s}}^{2}}\text{2}{{\text{p}}^{6}}\text{3}{{\text{s}}^{2}}\text{3}{{\text{p}}^{2}} \right)$ configuration. In the periodic table, it is placed before phosphorus. It follows general trends of periodic table. It has lower electronegativity than chlorine and sulphur in period 3.
The correct order of electronegativity is $\text{Cl}>\text{S}>\text{Si}>\text{Ar}$.
The correct answer to this question is option D.
Note:
Just by seeing the general trend that electronegativity increases from left to right, do not mark ‘$\text{Ar}$’ as the most electronegative element (being placed at the end of that period). Please take a note that $\text{Ar}$ is noble gas whose electronegativity is not counted.
Trend of electronegativity is as follows-

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




