
The monomers of BUNA-S rubber are:
A. Vinyl chloride and sulphur
B. Butadiene
C. Styrene and butadiene
D. Isoprene and Butadiene
Answer
570k+ views
Hint: BUNA-S is a copolymer containing two different monomeric units in its structure. It is a synthetic rubber prepared by using sodium as a reagent. BUNA-S comes under rubber due to its chemical and physical properties.
Complete step by step answer:
- In the question it is given that what are the monomers of the BUNA-S rubber.
- We have to write the monomeric units of the BUNA-S copolymer.
- The name BUNA-S itself reveals the monomers present in it.
- BU - stands for Butadiene, S- stands for Styrene, NA-stands for sodium.
- Means the monomeric units present in BUNA-S are butadiene and styrene.
- The structures of the butadiene and styrene are as follows.
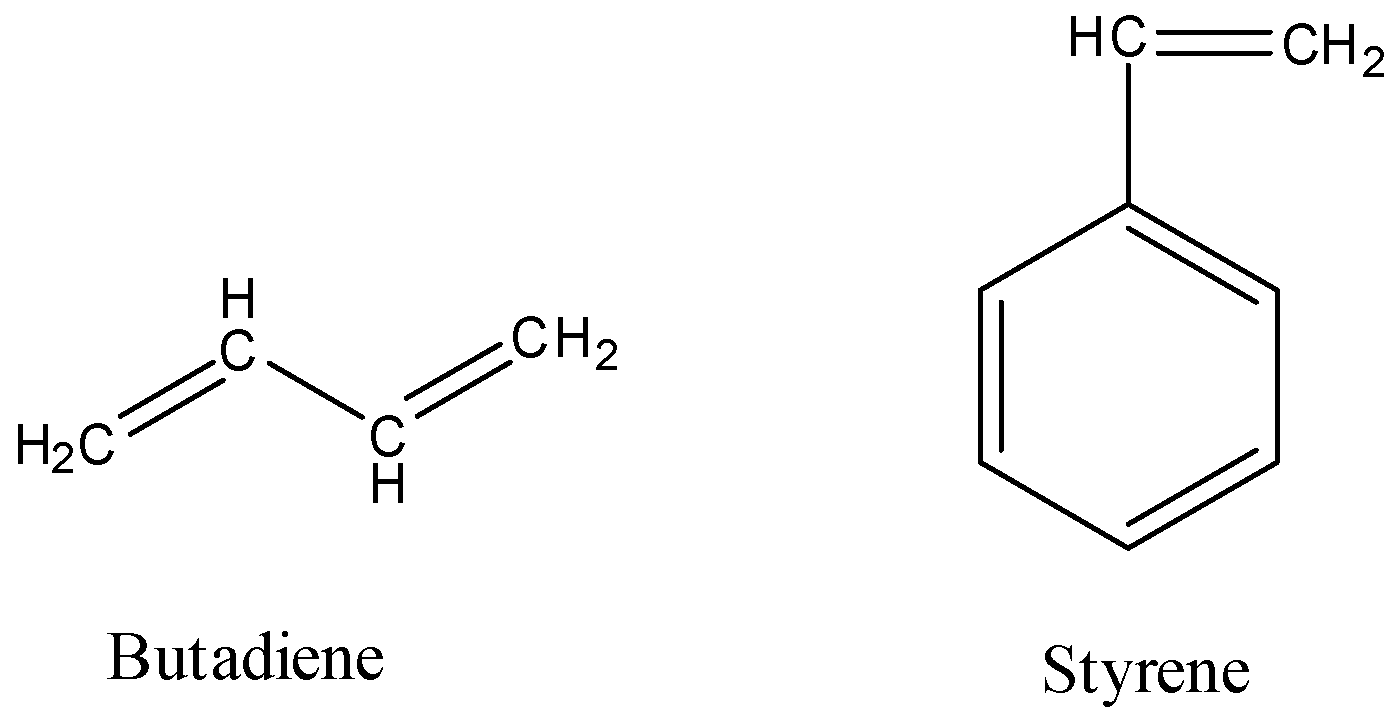
- The preparation of BUNA-S rubber from butadiene and styrene is as follows.
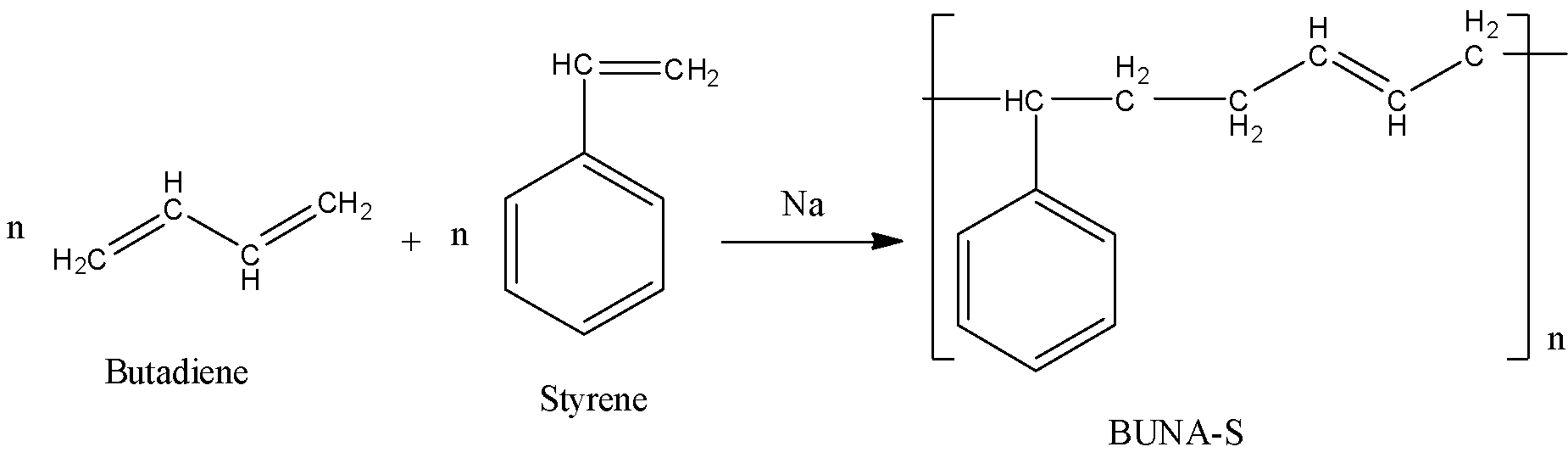
- In the above preparation, n moles of butadiene react with n moles of styrene and form the polymer BUNA-S as the product.
- Therefore BUNA-S contains Styrene and butadiene as monomeric units.
- So, the correct option is C.
Additional information:
- Due to its properties it competes with natural rubber.
- BUNA-S is mostly used in coating papers.
- It is a cost-effective resin.
- It is used in sealing as a binding agent and it is an alternative for PVA (poly vinyl alcohol).
Note: BUNA-S is going to form from its monomeric units (Styrene and butadiene) through addition polymerization reaction in the presence of Sodium. It is not a natural rubber, it is a synthetic rubber.
Complete step by step answer:
- In the question it is given that what are the monomers of the BUNA-S rubber.
- We have to write the monomeric units of the BUNA-S copolymer.
- The name BUNA-S itself reveals the monomers present in it.
- BU - stands for Butadiene, S- stands for Styrene, NA-stands for sodium.
- Means the monomeric units present in BUNA-S are butadiene and styrene.
- The structures of the butadiene and styrene are as follows.

- The preparation of BUNA-S rubber from butadiene and styrene is as follows.

- In the above preparation, n moles of butadiene react with n moles of styrene and form the polymer BUNA-S as the product.
- Therefore BUNA-S contains Styrene and butadiene as monomeric units.
- So, the correct option is C.
Additional information:
- Due to its properties it competes with natural rubber.
- BUNA-S is mostly used in coating papers.
- It is a cost-effective resin.
- It is used in sealing as a binding agent and it is an alternative for PVA (poly vinyl alcohol).
Note: BUNA-S is going to form from its monomeric units (Styrene and butadiene) through addition polymerization reaction in the presence of Sodium. It is not a natural rubber, it is a synthetic rubber.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




