
The monomers of Buna-S are:
A.Styrene and Butadiene
B.Isoprene and Butadiene
C.Vinyl chloride and Sulphur
D.Butadiene
Answer
601.2k+ views
Hint:
There are two monomers of Buna-S. One of the monomers is a simple conjugated diene with even number of double bonds at two positions. The other monomer is an organic gaseous compound which is poisonous in nature. It is a known carcinogen. The functional group attached to the benzene ring in this monomer is a vinyl group.
Complete step by step answer:
The monomers of Buna-S are styrene and butadiene.
The name Buna-S is derived from the combination of the following words-
Bu(Butadiene) + Na(Sodium) + S(Styrene)
It is a popular synthetic rubber. It is a great replacement of natural rubber.
It is a co-polymer formed by the polymerisation process of a mixture of 1:3 butadiene and styrene. Peroxide catalyst is present in the reaction under a temperature of \[{{5}^{\circ }}C\].In short it is also known as SBR.
The reaction of preparation of Buna-S is as follows:
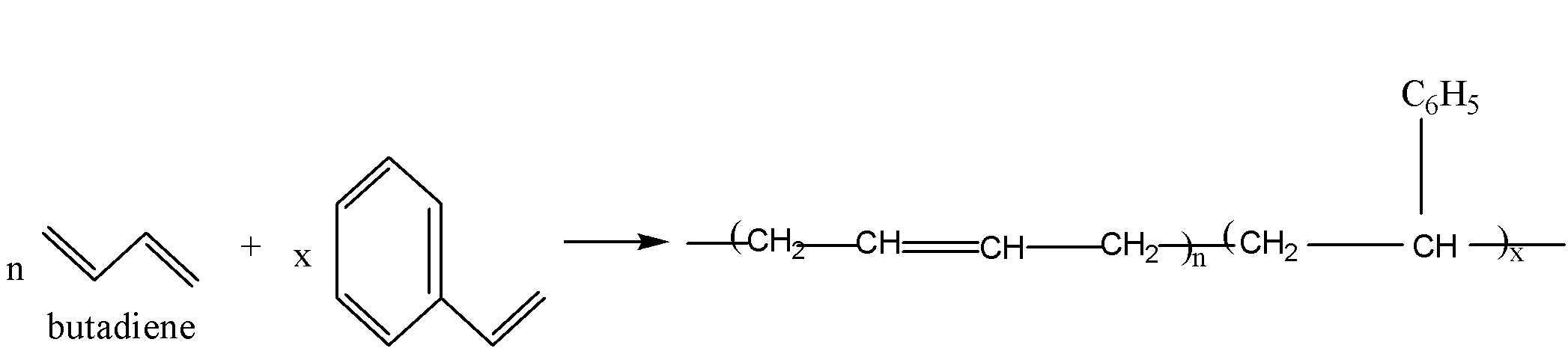
It is used for the manufacture of tyres and tubes of vehicles. It is also used for making shoe soles, conveyor belts and so on.
Chloroprene was the first commercially successful rubber manufactured.
So, the correct option is (A).
Note:
Synthetic rubber (Gutta-Percha) is made by the radical polymerization of isoprene. It has a trans configuration. So, it has a zigzag chain which cannot be stretched. This is the reason for the non-elasticity of the synthetic rubber.
There are two monomers of Buna-S. One of the monomers is a simple conjugated diene with even number of double bonds at two positions. The other monomer is an organic gaseous compound which is poisonous in nature. It is a known carcinogen. The functional group attached to the benzene ring in this monomer is a vinyl group.
Complete step by step answer:
The monomers of Buna-S are styrene and butadiene.
The name Buna-S is derived from the combination of the following words-
Bu(Butadiene) + Na(Sodium) + S(Styrene)
It is a popular synthetic rubber. It is a great replacement of natural rubber.
It is a co-polymer formed by the polymerisation process of a mixture of 1:3 butadiene and styrene. Peroxide catalyst is present in the reaction under a temperature of \[{{5}^{\circ }}C\].In short it is also known as SBR.
The reaction of preparation of Buna-S is as follows:

It is used for the manufacture of tyres and tubes of vehicles. It is also used for making shoe soles, conveyor belts and so on.
Chloroprene was the first commercially successful rubber manufactured.
So, the correct option is (A).
Note:
Synthetic rubber (Gutta-Percha) is made by the radical polymerization of isoprene. It has a trans configuration. So, it has a zigzag chain which cannot be stretched. This is the reason for the non-elasticity of the synthetic rubber.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




