
The monomeric unit of orlon molecule is:
(A) $C{{H}_{2}}=CH-Cl$
(B) $C{{H}_{3}}COO-CH=C{{H}_{2}}$
(C) $C{{H}_{2}}=CH-CN$
(D) ${{C}_{6}}{{H}_{5}}-CH=C{{H}_{2}}$
Answer
594k+ views
Hint: The chemical name of orlon is polyacrylonitrile. The monomeric unit contains a cyanide group.
Complete answer:
As mentioned in the hint, the chemical name of orlon is polyacrylonitrile, which means, it is a polymer of acrylonitrile. Now, it is very obvious that the formula of acrylonitrile contains a cyanide group. Its chemical formula is $C{{H}_{2}}=CH-CN$. The polymeric structure of orlon is as shown below:
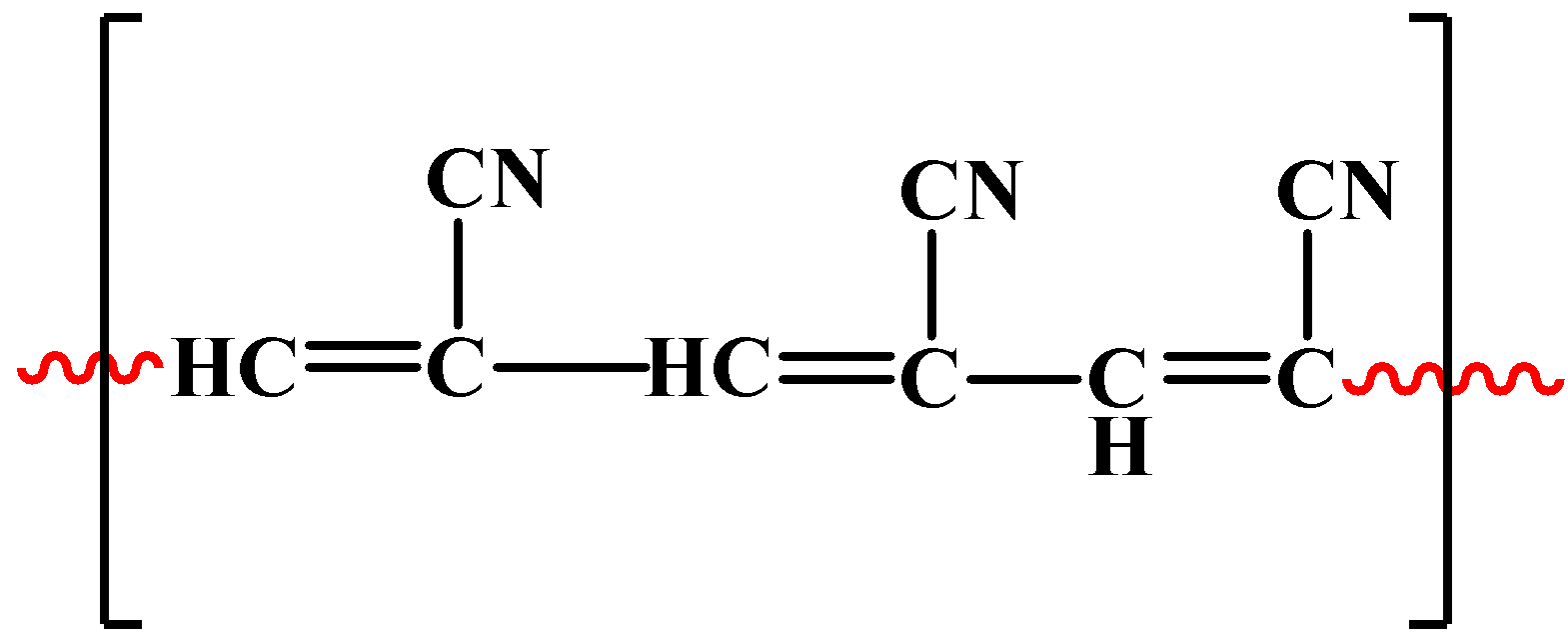
The chemical formula of this polymer is${{\left[ -CH=CCN- \right]}_{n}}$.
Additional Information:
- Orlon is a synthetic acrylic fibre. A fibre is a type of polymer which consists of many side-chains and branches. The high intermolecular attractions between their individual units due to forces like hydrogen bonds, make them stronger than normal polymers. Fibres have a crystalline structure because of the compact molecular arrangement. They are popular because of their high tensile strength which can be put into many different uses.
- Orlon is mainly used to make outdoor things such as awnings and other rooftop covers because it is resistant to sunlight and high temperatures.
- The tensile strength of orlon is not affected when it is wet. Therefore, it is used in making rainwear and other washable sportswear.
- Orlon is also resistant to chemicals, which makes it good for industrial apparels. Tough clothes are needed so that personnel damage due to harmful chemicals can be reduced.
- Orlon is also used in automobile industries. Here, it is used to produce the tops of vehicles.
Note: This question is easy to solve if you know that orlon is the polymer of acrylonitrile and that acrylonitrile has a cyanide group. It is because the chemical formula in other options does not contain the cyanide group. The question is difficult if you don’t have this information. It is therefore advised to memorise as much of the polymers and their monomeric units as you can.
Complete answer:
As mentioned in the hint, the chemical name of orlon is polyacrylonitrile, which means, it is a polymer of acrylonitrile. Now, it is very obvious that the formula of acrylonitrile contains a cyanide group. Its chemical formula is $C{{H}_{2}}=CH-CN$. The polymeric structure of orlon is as shown below:

The chemical formula of this polymer is${{\left[ -CH=CCN- \right]}_{n}}$.
Additional Information:
- Orlon is a synthetic acrylic fibre. A fibre is a type of polymer which consists of many side-chains and branches. The high intermolecular attractions between their individual units due to forces like hydrogen bonds, make them stronger than normal polymers. Fibres have a crystalline structure because of the compact molecular arrangement. They are popular because of their high tensile strength which can be put into many different uses.
- Orlon is mainly used to make outdoor things such as awnings and other rooftop covers because it is resistant to sunlight and high temperatures.
- The tensile strength of orlon is not affected when it is wet. Therefore, it is used in making rainwear and other washable sportswear.
- Orlon is also resistant to chemicals, which makes it good for industrial apparels. Tough clothes are needed so that personnel damage due to harmful chemicals can be reduced.
- Orlon is also used in automobile industries. Here, it is used to produce the tops of vehicles.
Note: This question is easy to solve if you know that orlon is the polymer of acrylonitrile and that acrylonitrile has a cyanide group. It is because the chemical formula in other options does not contain the cyanide group. The question is difficult if you don’t have this information. It is therefore advised to memorise as much of the polymers and their monomeric units as you can.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




