
The monomer of terylene are:
A. Phenol and formaldehyde
B. Ethylene glycol and phthalic acid
C. Adipic acid and hexamethylenediamine
D. Ethylene glycol and terephthalic acid
Answer
584.7k+ views
Hint: The structure of terylene consists of 1,4- dicarboxylic acid and ethane-1,2-diol. Dacron is the other name for terylene. Due to the presence of ester linkage, it is also known as polyester.
Complete Step-by-Step Answer:
-The structure of terylene is:
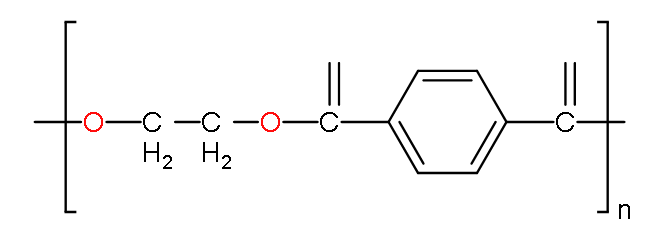
-In terylene, two main molecules bond together as a monomer by the process of polymerisation to form a polymer known as terylene.
-The monomers which polymerise are ethylene glycol and terephthalic acid.
-The molecular formula of ethylene glycol is $\text{nHO}{{\text{H}}_{2}}\text{C-C}{{\text{H}}_{2}}\text{OH}$
-The molecular formula of phthalic acid is \[\text{nHOOC-}{{\text{C}}_{6}}{{\text{H}}_{5}}\text{-COOH}\]
-The reaction of the formation of terylene is:

-They both react with each other and forms:
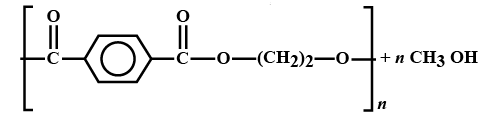
-So, option D. is an correct answer.
-Phenol and formaldehyde are the monomers which on polymerisation form Bakelite.
-So, option A. is an incorrect answer.
-Ethylene glycol and phthalic acid are the monomers which on polymerisation form Glyptal.
-So, option B. is also an incorrect answer.
-Adipic and hexamethylenediamine are the monomers which form polymerisation form Nylon-6,6.
-So, option C. is also an incorrect answer.
Therefore, option D. is the correct answer.
Note: The difference between polymer and monomers is that monomer is the smallest and repeating unit which bonds together and makes a long chain which is known as a polymer. For example, polythene is a long-chain polymer which is made from the ethene. Many molecules of ethene combine and make bonds to form a long chain of polythene. That’s why it is named as 'poly' which means 'many'.
Complete Step-by-Step Answer:
-The structure of terylene is:

-In terylene, two main molecules bond together as a monomer by the process of polymerisation to form a polymer known as terylene.
-The monomers which polymerise are ethylene glycol and terephthalic acid.
-The molecular formula of ethylene glycol is $\text{nHO}{{\text{H}}_{2}}\text{C-C}{{\text{H}}_{2}}\text{OH}$
-The molecular formula of phthalic acid is \[\text{nHOOC-}{{\text{C}}_{6}}{{\text{H}}_{5}}\text{-COOH}\]
-The reaction of the formation of terylene is:

-They both react with each other and forms:
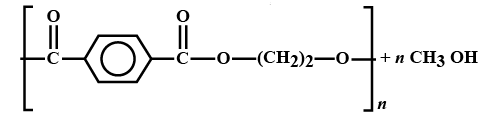
-So, option D. is an correct answer.
-Phenol and formaldehyde are the monomers which on polymerisation form Bakelite.
-So, option A. is an incorrect answer.
-Ethylene glycol and phthalic acid are the monomers which on polymerisation form Glyptal.
-So, option B. is also an incorrect answer.
-Adipic and hexamethylenediamine are the monomers which form polymerisation form Nylon-6,6.
-So, option C. is also an incorrect answer.
Therefore, option D. is the correct answer.
Note: The difference between polymer and monomers is that monomer is the smallest and repeating unit which bonds together and makes a long chain which is known as a polymer. For example, polythene is a long-chain polymer which is made from the ethene. Many molecules of ethene combine and make bonds to form a long chain of polythene. That’s why it is named as 'poly' which means 'many'.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




