
The molecule of Aspartame (Nutrasweet), depicted below, has …………… stereogenic centres.
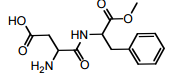
(A) 2
(B) 3
(C) 4
(D) 5

Answer
584.7k+ views
Hint: You should know that a stereogenic centre is also known as a chiral centre. It is characterized by an atom that has different groups bound to it in such a manner that its mirror image is non-superimposable. Now try to find stereogenic centres in Aspartame.
Complete step by step solution:
-Before answering this, let us discuss what a stereogenic centre is.
A carbon atom that is bonded to four different atoms or groups loses all symmetry and is often referred to as an asymmetric carbon and that carbon centre is known as the chiral or the stereogenic centre.
-Aspartame is an artificial non-saccharide sweetener. It is 200 times sweeter than sucrose. It is commonly used as a sugar substitute in foods and beverages.
It is basically a methyl ester of the aspartic acid/phenylalanine dipeptide with the trade names, NutraSweet. Aspartame is a methyl ester of the dipeptide of the natural amino acids L-aspartic acid and L-phenylalanine.
Now, let us see the structure and try to find the asymmetric centres.
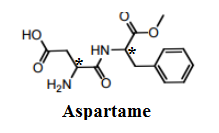
There are a total of two stereogenic centres in the Aspartame molecule. They are attached to four different atoms/groups and marked by an asterisk in the above image.
Therefore, we can conclude that the correct answer to this question is option (A)- 2.
Note: We should remember that optical isomers are compounds having the same atoms and bonds but in different spatial arrangements and will have non-superimposable mirror images. These non-superimposable mirror images are called enantiomers. However, the optical activity of a molecule does not depend solely on its chirality. If the molecule is dissymmetric and has at least one non-superimposable mirror image, it exhibits optical activity.
Complete step by step solution:
-Before answering this, let us discuss what a stereogenic centre is.
A carbon atom that is bonded to four different atoms or groups loses all symmetry and is often referred to as an asymmetric carbon and that carbon centre is known as the chiral or the stereogenic centre.
-Aspartame is an artificial non-saccharide sweetener. It is 200 times sweeter than sucrose. It is commonly used as a sugar substitute in foods and beverages.
It is basically a methyl ester of the aspartic acid/phenylalanine dipeptide with the trade names, NutraSweet. Aspartame is a methyl ester of the dipeptide of the natural amino acids L-aspartic acid and L-phenylalanine.
Now, let us see the structure and try to find the asymmetric centres.

There are a total of two stereogenic centres in the Aspartame molecule. They are attached to four different atoms/groups and marked by an asterisk in the above image.
Therefore, we can conclude that the correct answer to this question is option (A)- 2.
Note: We should remember that optical isomers are compounds having the same atoms and bonds but in different spatial arrangements and will have non-superimposable mirror images. These non-superimposable mirror images are called enantiomers. However, the optical activity of a molecule does not depend solely on its chirality. If the molecule is dissymmetric and has at least one non-superimposable mirror image, it exhibits optical activity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




