
What will be the major product, which can be obtained from the following reaction?
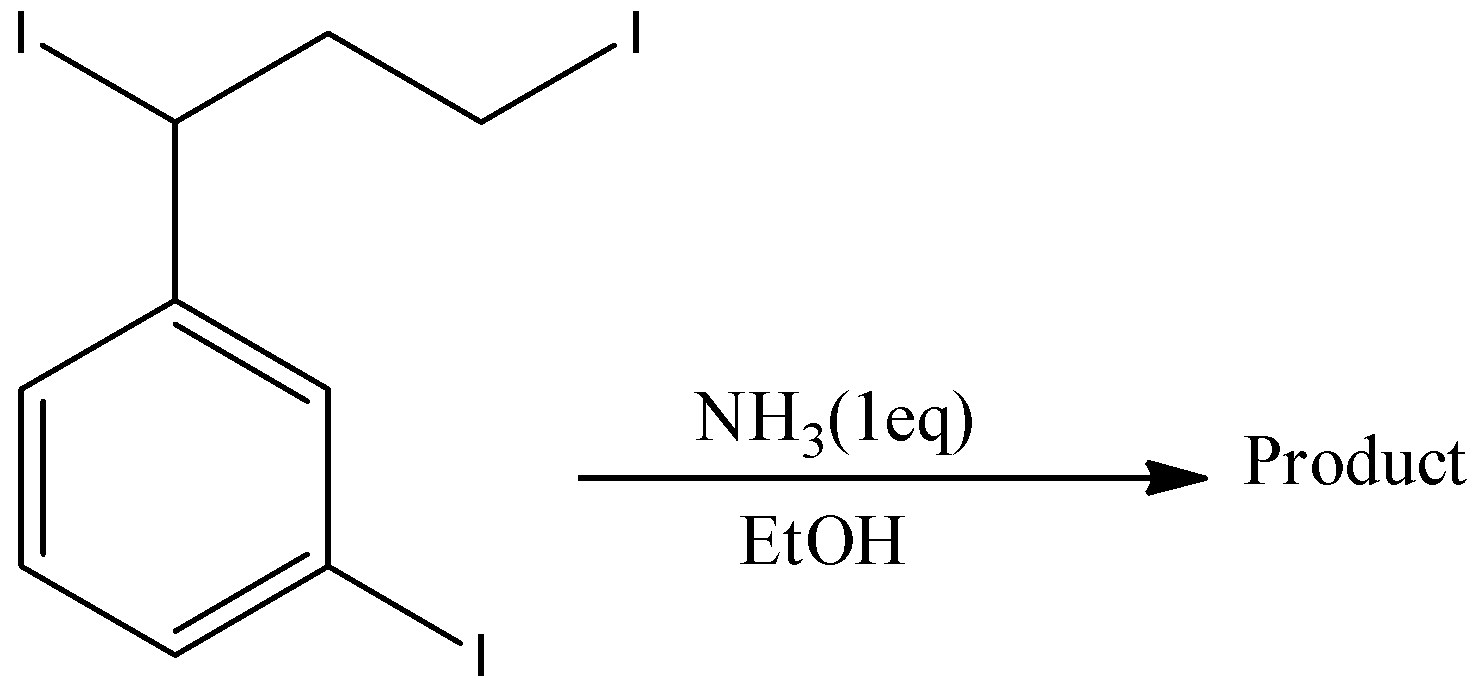
A.
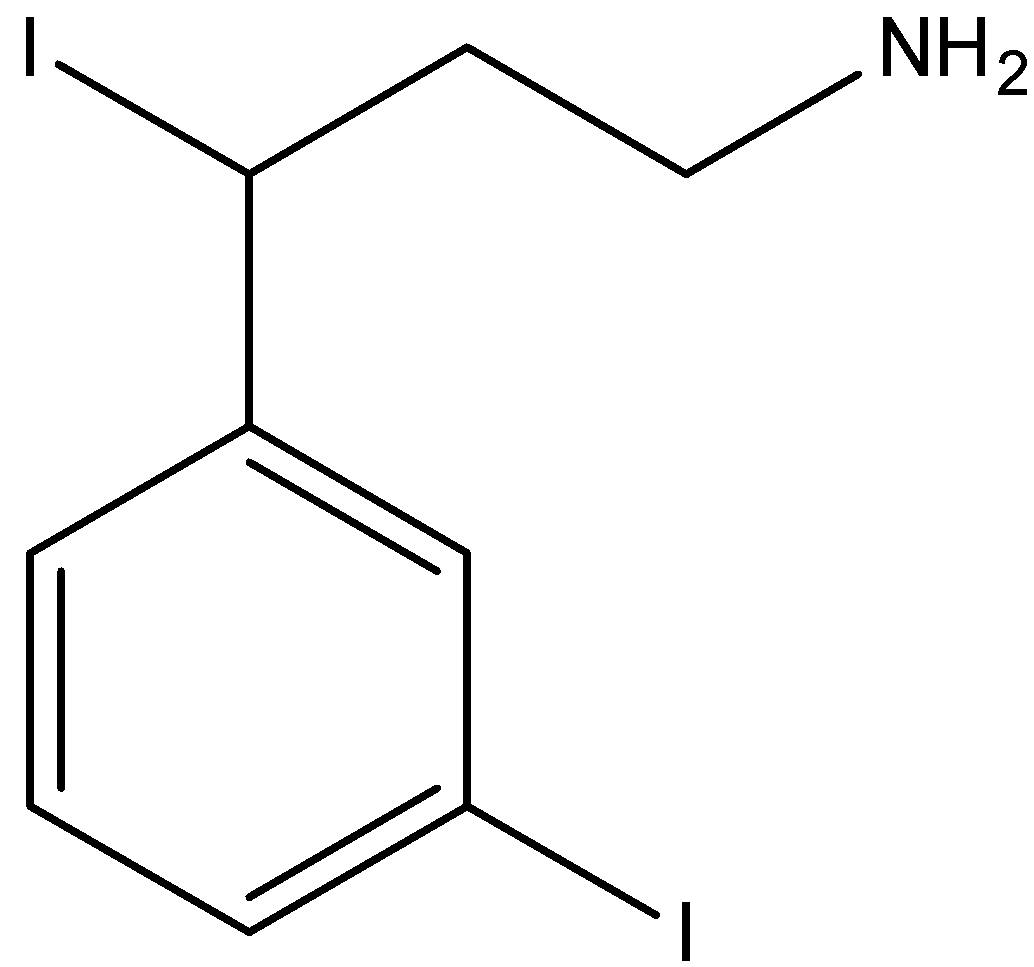
B.
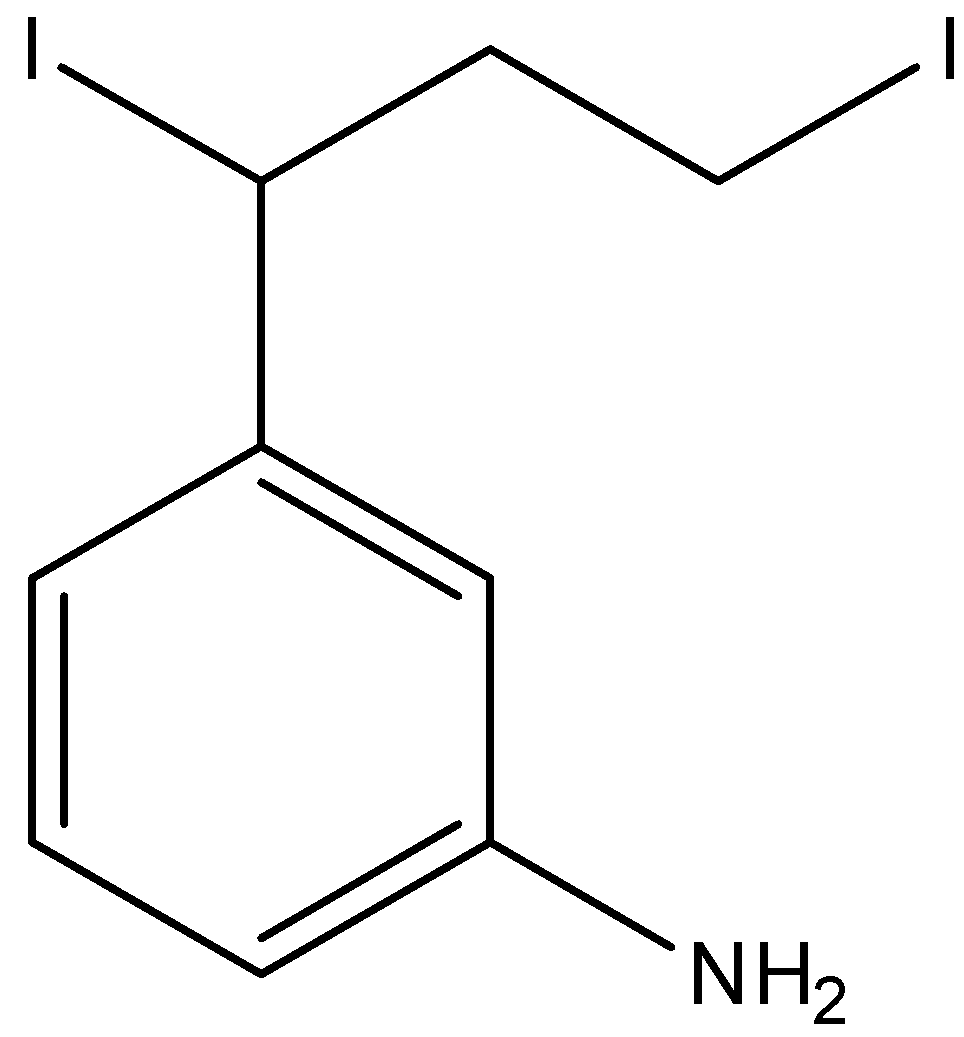
C.
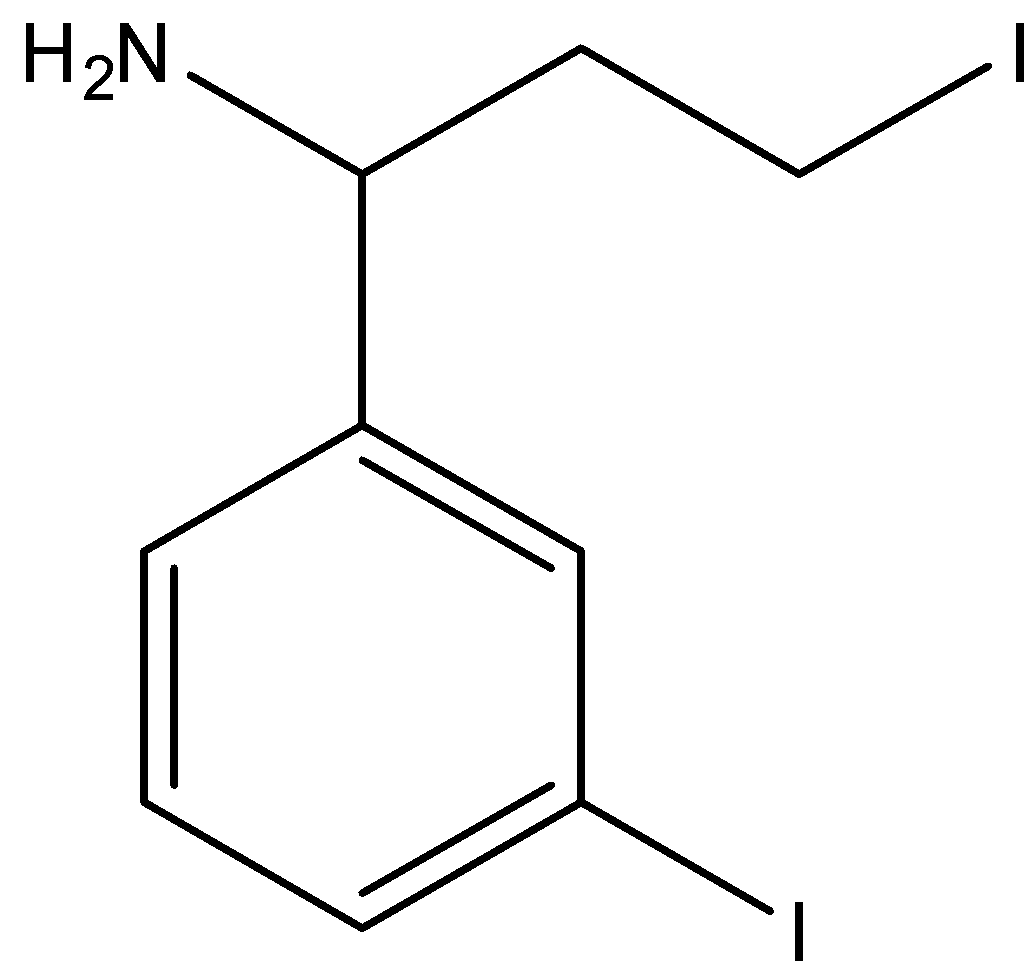
D.





Answer
558.9k+ views
Hint: Aromatic halides react with ammonia and produce respective amine derivatives as the products. The reaction of amine with aromatic halides in the presence of alcohol is called nucleophilic substitution reaction.
Complete step by step answer:
- In the question it is asked after completion of the given reaction what is the major product formed among the given options.
- The given chemical reaction is as follows.
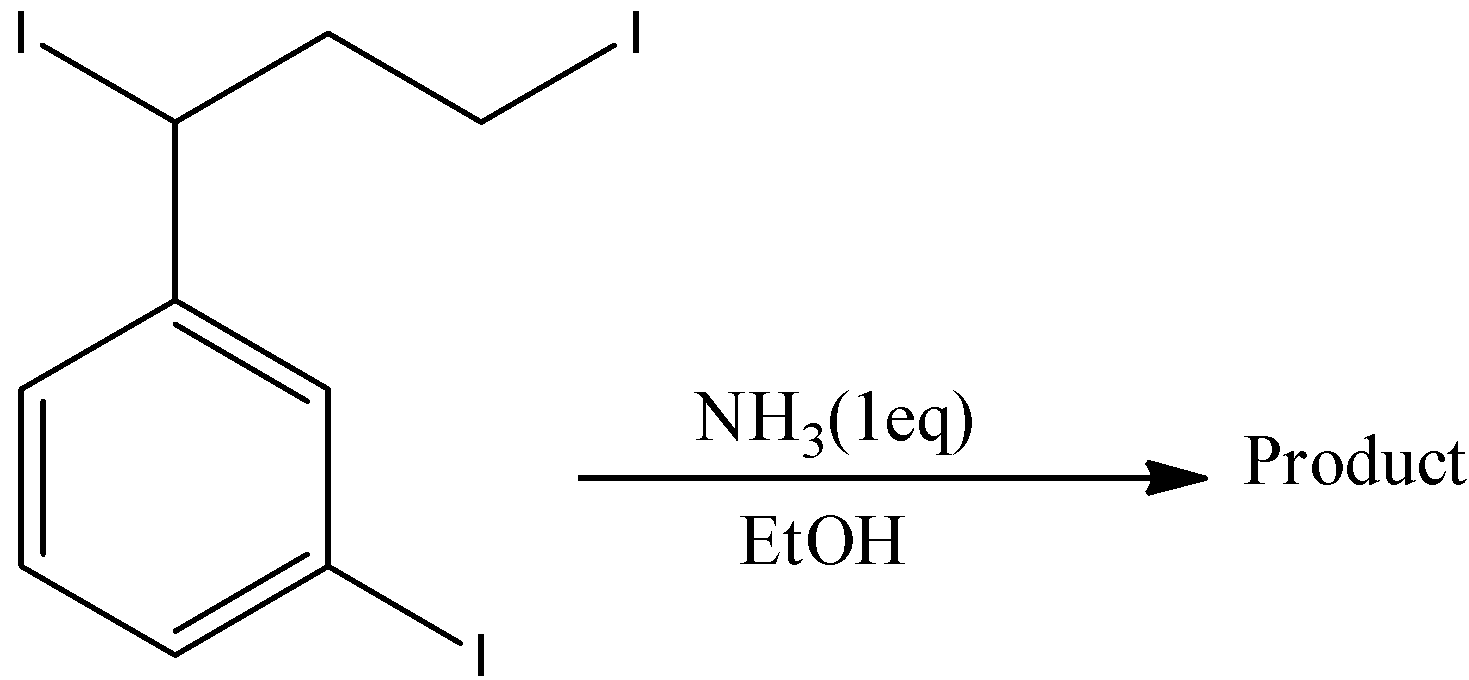
- In the reactant there are three types of iodine present.
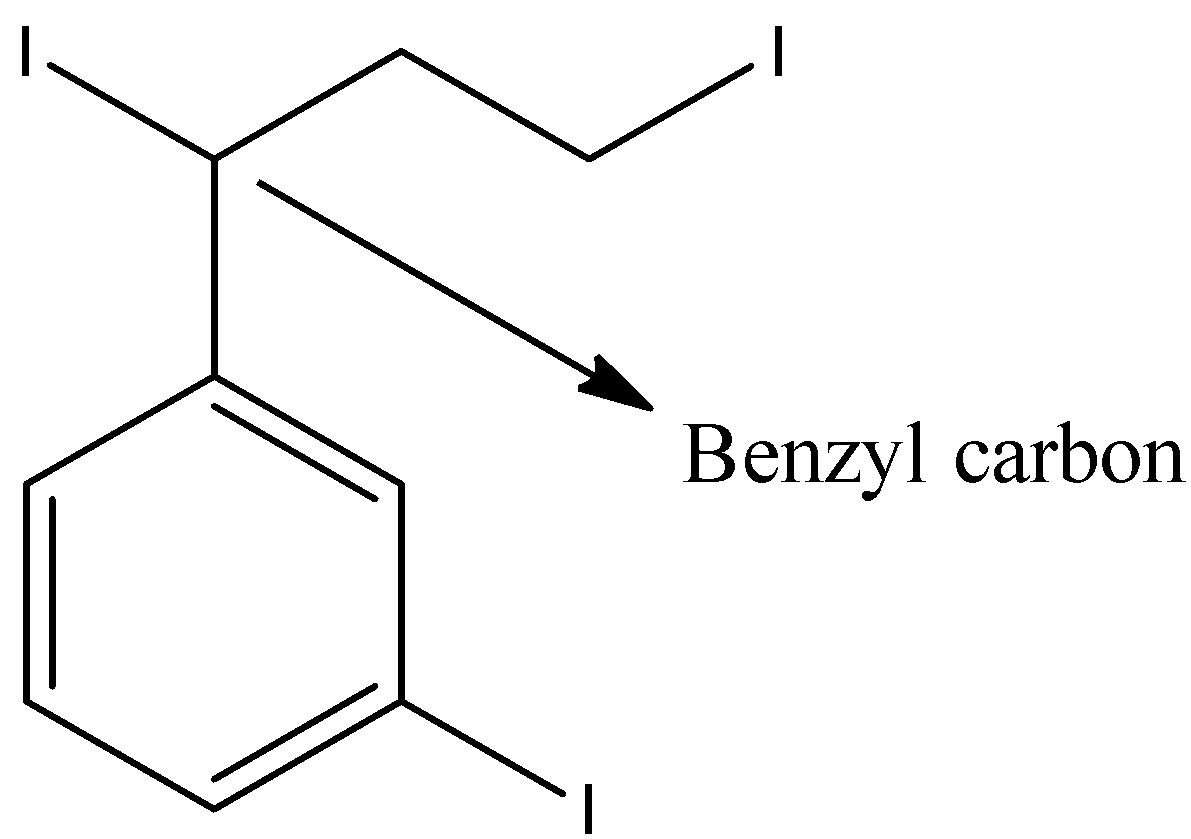
- First iodine is attached to the benzene ring directly, second iodine is attached to benzylic carbon and the third iodine is attached at the end of the alkyl chain.
- We have to find out at which place the ammonia is going to attack.
- We know that it is very difficult to remove the halogen which is attached directly to the benzene ring then the iodine attached directly to the benzene ring does not react with ammonia.
- Now the iodine which is attached at the end of the alkyl chain reacts with ammonia but a very small amount of the product is going to form.
- Coming to iodine which is at benzylic carbon undergoes nucleophilic substitution reaction with ammonia and forms an amine derivative in major amounts due to the presence of partial positive at benzylic carbon.
- The partial positive charge at benzylic carbon is due to the presence of electron withdrawing halogen and the benzene ring also takes electrons from the benzylic carbon.
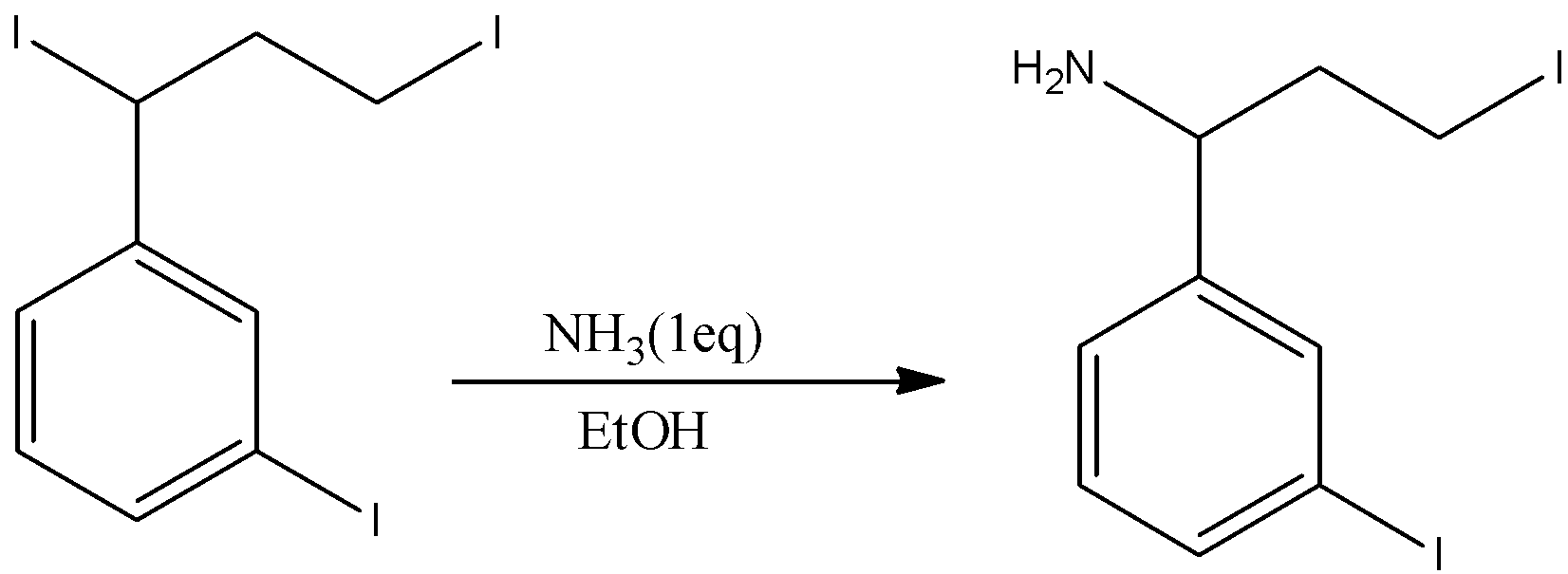
- The chemical reaction of ammonia with the given reactant is as follows.
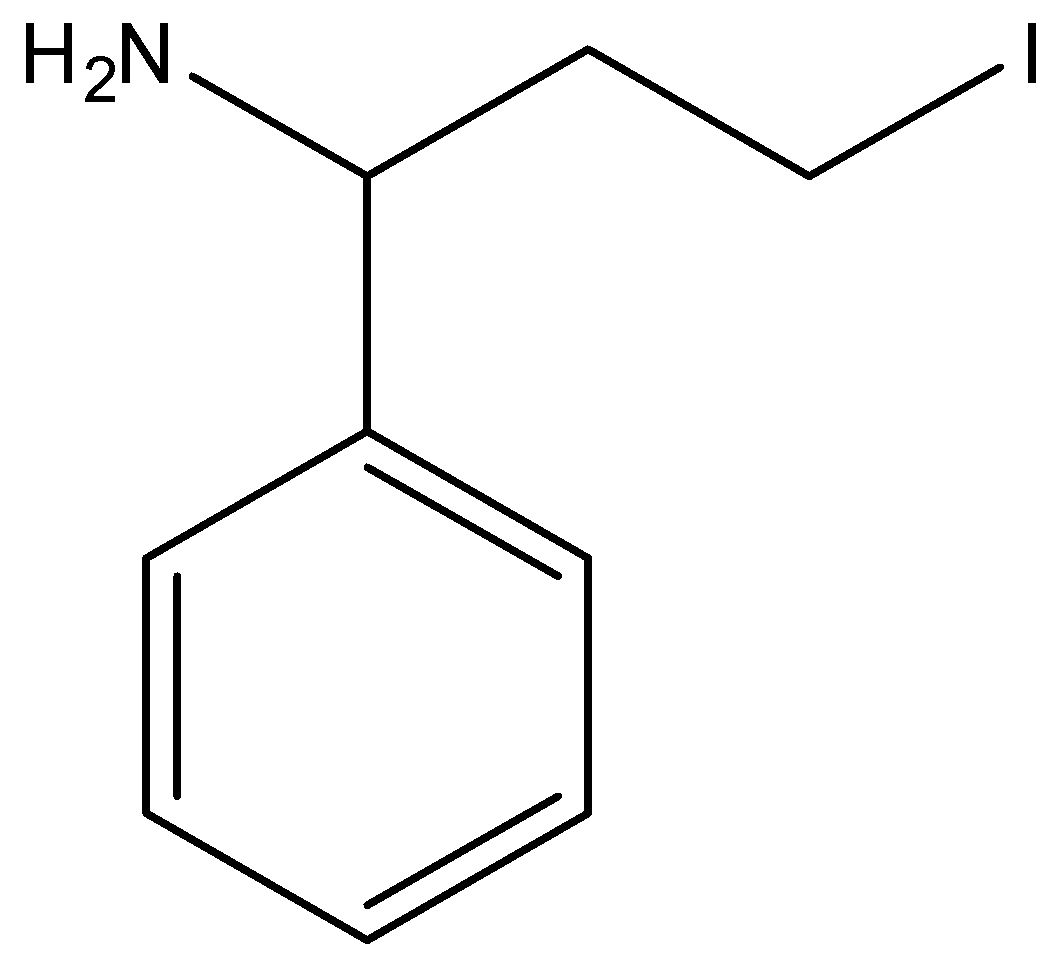
The correct option is option “C” .
Note: Ammonia is a good nucleophile and it reacts with highly reactive halogens and forms respective amines as the products. This reaction is an example of a substitution reaction. In industries alkyl or aryl amines are going to prepare in this manner.
Complete step by step answer:
- In the question it is asked after completion of the given reaction what is the major product formed among the given options.
- The given chemical reaction is as follows.

- In the reactant there are three types of iodine present.
- First iodine is attached to the benzene ring directly, second iodine is attached to benzylic carbon and the third iodine is attached at the end of the alkyl chain.
- We have to find out at which place the ammonia is going to attack.
- We know that it is very difficult to remove the halogen which is attached directly to the benzene ring then the iodine attached directly to the benzene ring does not react with ammonia.
- Now the iodine which is attached at the end of the alkyl chain reacts with ammonia but a very small amount of the product is going to form.
- Coming to iodine which is at benzylic carbon undergoes nucleophilic substitution reaction with ammonia and forms an amine derivative in major amounts due to the presence of partial positive at benzylic carbon.
- The partial positive charge at benzylic carbon is due to the presence of electron withdrawing halogen and the benzene ring also takes electrons from the benzylic carbon.

- The chemical reaction of ammonia with the given reactant is as follows.

The correct option is option “C” .
Note: Ammonia is a good nucleophile and it reacts with highly reactive halogens and forms respective amines as the products. This reaction is an example of a substitution reaction. In industries alkyl or aryl amines are going to prepare in this manner.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




