
What would be the major product of the following reaction?
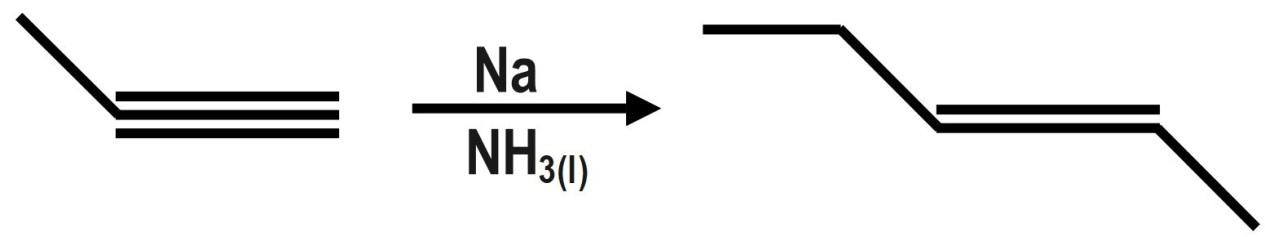
A.
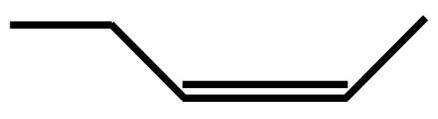
B.
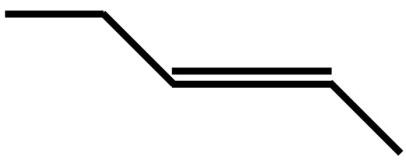
C.

D.






Answer
513.3k+ views
Hint: We know that to solve given problem, we should have understanding about alkynes, Birch reduction and Cis and Trans alkenes. Alkynes are unsaturated hydrocarbons containing at least one carbon - carbon triple bond. They have a general chemical formula ${{C}_{n}}{{H}_{2n-2}}$ . Birch reduction is an organic reaction where the reagents used are Sodium and liquid ammonia on the reactant alkyne to produce alkene products.
Complete answer: Let us first discuss the Birch reduction: The Birch reduction is an organic chemical reaction where aromatic compounds. Conjugated enamines can also be formed from the Birch reduction of aniline. Alkynes can also undergo Birch reduction to form alkenes. Birch reduction is named after the Australian chemist Arthur Birch. The reaction type involved in this process is organic redox reaction. -Alkynes are selectively converted into Trans alkenes when they are reduced by a solution of sodium (or lithium) in liquid that contains stoichiometric amounts of an alcohol such as ethanol.
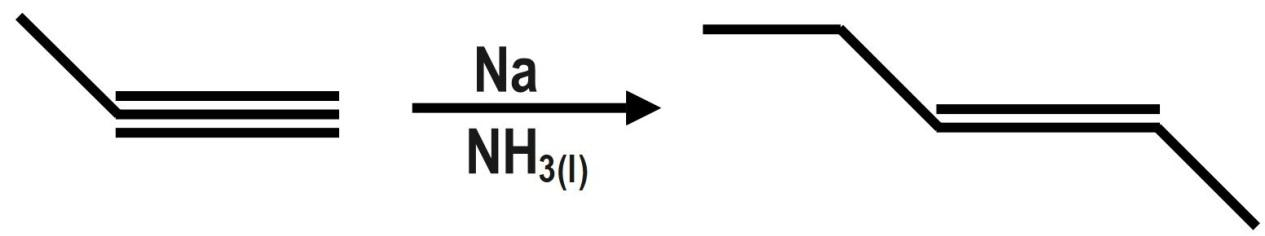
In the given question, we have line annotation of the organic compound alkyne. So, we convert it to the carbon and hydrogen form. When we react with the given reagent with sodium and liquid ammonia, birch reduction takes place and a trans product is formed. The Trans product has to be converted back to line annotation. Also the same branched reaction can be given as;
\[{{H}_{3}}C-C{{H}_{2}}-{{C}_{2}}-C{{H}_{3}}\xrightarrow[N{{H}_{3}}_{(l)}]{Na}{{H}_{5}}{{C}_{2}}-CH=CH-C{{H}_{3}}\]
Therefore, the correct option is B.
Additional Information: While cis isomers are generally polar, the same cannot be said for Tran’s forms as they are generally referred to as non - polar molecules. The melting point of cis isomer are relatively lesser than Trans because of the packaging of molecules is tightly in trans form. The boiling point of cis is greater than trans isomers because of strong attractive forces in cis isomer. Cis and Trans are the geometrical isomers. In cis isomers, same groups are present on the same side of double bond while in Trans, the group on one side of isomer is different.
Note:
Remember that while doing the conversion reactions in organic chemistry, we should pay specific attention to the reagents as smaller changes in them can change the products completely. The conversion of alkyne to trans-alkene by Birch reduction using alkali metals (such as \[~Na\] or \[K\] ) in liquid and alcohol \[\left( MeOH\text{ }or\text{ }EtOH \right),\] four radicals are formed i.e. radical anion, vinylic radical, trans-vinylic anion, trans alkene.
Complete answer: Let us first discuss the Birch reduction: The Birch reduction is an organic chemical reaction where aromatic compounds. Conjugated enamines can also be formed from the Birch reduction of aniline. Alkynes can also undergo Birch reduction to form alkenes. Birch reduction is named after the Australian chemist Arthur Birch. The reaction type involved in this process is organic redox reaction. -Alkynes are selectively converted into Trans alkenes when they are reduced by a solution of sodium (or lithium) in liquid that contains stoichiometric amounts of an alcohol such as ethanol.

In the given question, we have line annotation of the organic compound alkyne. So, we convert it to the carbon and hydrogen form. When we react with the given reagent with sodium and liquid ammonia, birch reduction takes place and a trans product is formed. The Trans product has to be converted back to line annotation. Also the same branched reaction can be given as;
\[{{H}_{3}}C-C{{H}_{2}}-{{C}_{2}}-C{{H}_{3}}\xrightarrow[N{{H}_{3}}_{(l)}]{Na}{{H}_{5}}{{C}_{2}}-CH=CH-C{{H}_{3}}\]
Therefore, the correct option is B.
Additional Information: While cis isomers are generally polar, the same cannot be said for Tran’s forms as they are generally referred to as non - polar molecules. The melting point of cis isomer are relatively lesser than Trans because of the packaging of molecules is tightly in trans form. The boiling point of cis is greater than trans isomers because of strong attractive forces in cis isomer. Cis and Trans are the geometrical isomers. In cis isomers, same groups are present on the same side of double bond while in Trans, the group on one side of isomer is different.
Note:
Remember that while doing the conversion reactions in organic chemistry, we should pay specific attention to the reagents as smaller changes in them can change the products completely. The conversion of alkyne to trans-alkene by Birch reduction using alkali metals (such as \[~Na\] or \[K\] ) in liquid and alcohol \[\left( MeOH\text{ }or\text{ }EtOH \right),\] four radicals are formed i.e. radical anion, vinylic radical, trans-vinylic anion, trans alkene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




