
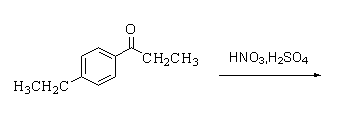
The major organic product of the following reaction.

Answer
576.3k+ views
Hint:In this reaction nitration takes place. Nitration is a process in which a proton is replaced by a nitro group formed by the reaction between nitric acid and sulphuric acid.
Complete step by step answer:
-Halogenation, sulfonation and nitration are types of electrophilic substitution reactions which are shown by aromatic aldehyde and ketones.
-In the above reaction we are discussing nitration with aromatic ketone.
-First step deals with the generation of electrophile in which nitric acid and sulphuric acid react to form nitronium ion which acts as an electrophile in nitration reaction. And since aromatic ketones are deactivating group ,meta-directing and electron withdrawing group In aromatic electrophilic substitution substituent that favour meta electrophilic attack to the substituent are called meta director so the proton from meta position which will act as a nucleophile and attack on the nitro group which is been liberated in the first step of the reaction to form meta directing compound.
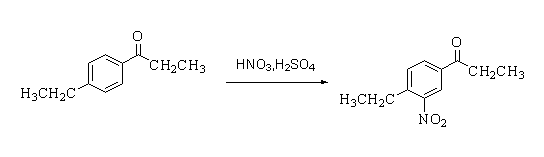
-In this reaction, starting material 1-(4-ethylphenyl)propan-1-one is converted to 1-(4-ethyl-3-nitrophenyl)propan-1-one in presence of nitric acid and sulphuric acid.
Note:
One of the other reason of nitro group being meta director is that if we have a look at the resonance structure then it shows that concentration of positive charge have increased at the ortho and para position due to the presence of nitro group thus making these ortho and para position deactivated for aromatic electrophilic substitution .hence nitro is a meta director.
Complete step by step answer:
-Halogenation, sulfonation and nitration are types of electrophilic substitution reactions which are shown by aromatic aldehyde and ketones.
-In the above reaction we are discussing nitration with aromatic ketone.
-First step deals with the generation of electrophile in which nitric acid and sulphuric acid react to form nitronium ion which acts as an electrophile in nitration reaction. And since aromatic ketones are deactivating group ,meta-directing and electron withdrawing group In aromatic electrophilic substitution substituent that favour meta electrophilic attack to the substituent are called meta director so the proton from meta position which will act as a nucleophile and attack on the nitro group which is been liberated in the first step of the reaction to form meta directing compound.
-In this reaction, starting material 1-(4-ethylphenyl)propan-1-one is converted to 1-(4-ethyl-3-nitrophenyl)propan-1-one in presence of nitric acid and sulphuric acid.

Note:
One of the other reason of nitro group being meta director is that if we have a look at the resonance structure then it shows that concentration of positive charge have increased at the ortho and para position due to the presence of nitro group thus making these ortho and para position deactivated for aromatic electrophilic substitution .hence nitro is a meta director.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




