
The IUPAC name of $ {({C_2}{H_5})_2}CHC{H_2}OH $ is
(A) $ 2 - ethyl - 1 - butanol $
(B) $ 2 - methyl - 1 - pentanol $
(C) $ 2 - ethyl - 1 - pentanol $
(D) $ 3 - ethyl - 1 - butanol $
Answer
506.7k+ views
Hint: We know that there are some rules to give name to an organic or inorganic compound which are given by the IUPAC (International Union of Pure and Applied Chemistry). These rules are followed very strictly while naming the chemical compounds. So, we will first look at the structure of the given organic compound and then follow the rules to give its IUPAC name.
Complete Step By Step Answer:
Let us first see the structure of the given chemical compound $ {({C_2}{H_5})_2}CHC{H_2}OH $ :
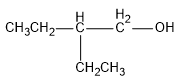
- First, we will identify the longest chain in the given compound. Therefore, in the given compound, the longest alkyl chain is butane because it has four carbon atoms.
- Now, we give numbers to the carbons of this longest chain. The numbers are given from the functional group with greater priority than the rest of the functional groups present in the compound. Here, only the alcoholic group is present in the given compound so number $ 1 $ will be given to the carbon attached to the alcoholic group. Now, the suffix “-ol” is added to the name of the longest chain.
At the second position of the carbon chain, an ethyl group is present.
Hence, we can conclude the IUPAC name of the given compound as $ 2 - ethyl - 1 - butanol $ .
Therefore, the correct option is (A) $ 2 - ethyl - 1 - butanol $ .
Note:
We should remember that while numbering the longest carbon chain the lower number should be given to that functional group which has higher priority than the other functional groups present in the organic compound. The priority order is as follows Carboxylic acid> Aldehyde> Ketone> Alcohol> Alkyne> Alkene> Alkane.
Complete Step By Step Answer:
Let us first see the structure of the given chemical compound $ {({C_2}{H_5})_2}CHC{H_2}OH $ :

- First, we will identify the longest chain in the given compound. Therefore, in the given compound, the longest alkyl chain is butane because it has four carbon atoms.
- Now, we give numbers to the carbons of this longest chain. The numbers are given from the functional group with greater priority than the rest of the functional groups present in the compound. Here, only the alcoholic group is present in the given compound so number $ 1 $ will be given to the carbon attached to the alcoholic group. Now, the suffix “-ol” is added to the name of the longest chain.
At the second position of the carbon chain, an ethyl group is present.
Hence, we can conclude the IUPAC name of the given compound as $ 2 - ethyl - 1 - butanol $ .
Therefore, the correct option is (A) $ 2 - ethyl - 1 - butanol $ .
Note:
We should remember that while numbering the longest carbon chain the lower number should be given to that functional group which has higher priority than the other functional groups present in the organic compound. The priority order is as follows Carboxylic acid> Aldehyde> Ketone> Alcohol> Alkyne> Alkene> Alkane.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




