
The hydrides of the first element in the group 15-17, namely \[N{H_3},{\text{ }}{H_2}O\] and \[HF,\] respectively show abnormally high values for melting and boiling points. This is due to:
A.Small size of N, O , and F
B.The ability to form extensive intermolecular H- bonding
C.The ability to form extensive intramolecular H- bonding
D.Effective van der Waals interaction
Answer
594k+ views
Hint: Hydrogen bonding interaction involves a hydrogen atom located between a pair of other atoms having a high affinity for electrons.
Complete step by step answer:
-The above reason is due to the ability to form extensive intermolecular H-bonding.
-This is because of the small size and high electronegativity of elements, have the ability to form extensive intermolecular (i.e. between two molecules) hydrogen bonding
-Thus, a large amount of energy is required to break these bonds i.e. the melting and boiling points of hydrides of these elements are abnormally high.
-The interaction between molecules is much stronger when there are intermolecular hydrogen bonds because the bonds are formed between molecules.
-Further, the strength of hydrogen bond depends upon the coulombic interactions between the electronegativity of the attached atom and hydrogen.
-It is a special type of dipole-dipole attraction between molecules.
Intermolecular hydrogen bonding is as shown:
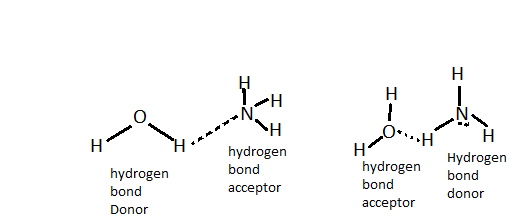
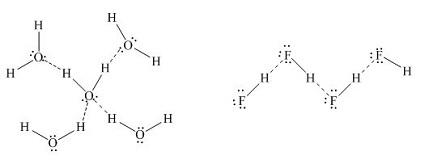
Hence, option B is correct.
Note:
Dipole- dipole interactions are the strongest intermolecular interactions and the ionic bond is the weakest of the true chemical bonds that bind atoms to atoms.
Complete step by step answer:
-The above reason is due to the ability to form extensive intermolecular H-bonding.
-This is because of the small size and high electronegativity of elements, have the ability to form extensive intermolecular (i.e. between two molecules) hydrogen bonding
-Thus, a large amount of energy is required to break these bonds i.e. the melting and boiling points of hydrides of these elements are abnormally high.
-The interaction between molecules is much stronger when there are intermolecular hydrogen bonds because the bonds are formed between molecules.
-Further, the strength of hydrogen bond depends upon the coulombic interactions between the electronegativity of the attached atom and hydrogen.
-It is a special type of dipole-dipole attraction between molecules.
Intermolecular hydrogen bonding is as shown:


Hence, option B is correct.
Note:
Dipole- dipole interactions are the strongest intermolecular interactions and the ionic bond is the weakest of the true chemical bonds that bind atoms to atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




