
The hybridization of $P$ in $POC{l_3}$ and shape of $POC{l_3}$ are, respectively
A.$s{p^3}$, tetrahedral
B.$s{p^3}$, pyramidal
C.$s{p^3}$, square planar
D.$s{p^3}$, distorted tetrahedral
Answer
527k+ views
Hint: At first you should know about hybridization and the process to draw the structures of compounds. Hybridization is defined as the concept of mixing two atomic orbitals with the same energy levels to give new degenerate orbitals.
Complete answer:
In order to determine the hybridization, we need to draw the structure of $POC{l_3}$. To form a bond between the two atoms there should be sharing of electron pairs between the atoms. As we all know that chlorine has one lone pair and oxygen has two lone pairs of atoms, the phosphorus atom forms three single bonds with three chlorine atoms and a double with one oxygen atom.
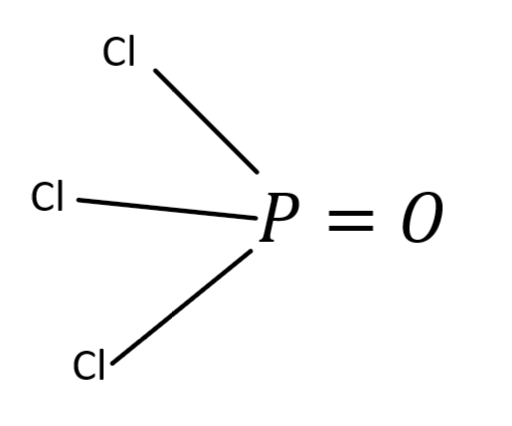
From the above diagram, we can say that P has $3\sigma $ bonds with three chlorine atoms and it formed a double bond with an oxygen atom. We all know that a double bond contains one $\sigma $ and one $\pi $ bond.
Hybridization of an atom can also be calculated by using no of $\sigma $bonds that the atom is attached to. So from the above diagram the P atom is attached to $4{\sigma _{}}$ bonds then the hybridization of P atom is $s{p^3}$.
The shape of $POC{l_3}$is tetrahedral due to the asymmetric charge distribution around the phosphorus atom.
So the answer is A.
Note:
To draw the structures of different compounds you should keep in mind about the lone pairs of atoms and its electronic configuration. Without knowing about the electronic configuration you cannot have a basic idea of valence electrons.
Complete answer:
In order to determine the hybridization, we need to draw the structure of $POC{l_3}$. To form a bond between the two atoms there should be sharing of electron pairs between the atoms. As we all know that chlorine has one lone pair and oxygen has two lone pairs of atoms, the phosphorus atom forms three single bonds with three chlorine atoms and a double with one oxygen atom.

From the above diagram, we can say that P has $3\sigma $ bonds with three chlorine atoms and it formed a double bond with an oxygen atom. We all know that a double bond contains one $\sigma $ and one $\pi $ bond.
Hybridization of an atom can also be calculated by using no of $\sigma $bonds that the atom is attached to. So from the above diagram the P atom is attached to $4{\sigma _{}}$ bonds then the hybridization of P atom is $s{p^3}$.
The shape of $POC{l_3}$is tetrahedral due to the asymmetric charge distribution around the phosphorus atom.
So the answer is A.
Note:
To draw the structures of different compounds you should keep in mind about the lone pairs of atoms and its electronic configuration. Without knowing about the electronic configuration you cannot have a basic idea of valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




