
The hcp and ccp structures for a given element would be expected to have:
(A) Same coordinator number
(B) Same density if made up of small element
(C) Same packing fraction
(D) Same number of atoms in single unit cell
Answer
588.6k+ views
Hint: The ordered structure that has occurred from the intrinsic nature of the constituent particles or atoms to form symmetric patterns of the crystal structure that repeat along all the dimensions are known as unit cells.
Complete step by step answer:
In a Hexagonal close packed structure (hcp) the third layer of the atoms has the same arrangement as per the spheres of the first layer while the second layer covers are different. Thus, it can be represented as ‘ababab…’ The spheres or atoms of the third layer covers all the octahedral holes.
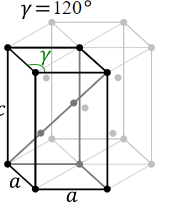
Diagram showing HCP structure
Whereas, in the cubic close packed (ccp) structure, the second & the third layer is used to cover the depressions of first and second layer respectively. As all these three layers are completely different from each other thus it can be represented as ‘ABC ABC…’.
Coordination number is used to represent the number of atoms that touch a particular atom. Both the hcp & ccp structures have a coordination number of $\left( {\alpha ,\beta ,\gamma } \right)$ $12$.
The hcp and ccp structure cannot have the same density even if they both are made up of the same element because both these structures have different numbers of atoms per unit cell. The hcp structure has $8$ spheres per unit cell whereas the ccp structure has $4$ spheres per unit cell.
Both the hcp & ccp structures have a packing efficiency of $74$ percent. Thus, the packing fraction would also be the same i.e. both the structures have the packing fraction of $0.74$.
As we know that the hcp and ccp structure has $8$ and $4$ atoms per unit cell respectively. Therefore, this option is not true.
Hence, the answer is option (A) and option (C).
Note:
The Geometry of the unit cell is defined as the parallelepiped, which give us the six lattice parameters that are the lengths of the edges of the cell (a, b, c), i.e. the length, breadth and height and the angles $\left( {\alpha ,\beta ,\gamma } \right)$ between these lengths.
Complete step by step answer:
In a Hexagonal close packed structure (hcp) the third layer of the atoms has the same arrangement as per the spheres of the first layer while the second layer covers are different. Thus, it can be represented as ‘ababab…’ The spheres or atoms of the third layer covers all the octahedral holes.

Diagram showing HCP structure
Whereas, in the cubic close packed (ccp) structure, the second & the third layer is used to cover the depressions of first and second layer respectively. As all these three layers are completely different from each other thus it can be represented as ‘ABC ABC…’.
Coordination number is used to represent the number of atoms that touch a particular atom. Both the hcp & ccp structures have a coordination number of $\left( {\alpha ,\beta ,\gamma } \right)$ $12$.
The hcp and ccp structure cannot have the same density even if they both are made up of the same element because both these structures have different numbers of atoms per unit cell. The hcp structure has $8$ spheres per unit cell whereas the ccp structure has $4$ spheres per unit cell.
Both the hcp & ccp structures have a packing efficiency of $74$ percent. Thus, the packing fraction would also be the same i.e. both the structures have the packing fraction of $0.74$.
As we know that the hcp and ccp structure has $8$ and $4$ atoms per unit cell respectively. Therefore, this option is not true.
Hence, the answer is option (A) and option (C).
Note:
The Geometry of the unit cell is defined as the parallelepiped, which give us the six lattice parameters that are the lengths of the edges of the cell (a, b, c), i.e. the length, breadth and height and the angles $\left( {\alpha ,\beta ,\gamma } \right)$ between these lengths.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
What are the major means of transport Explain each class 12 social science CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE

Calculate the equivalent resistance between a and b class 12 physics CBSE

How many states of matter are there in total class 12 chemistry CBSE

Which of the following is the best conductor of electricity class 12 physics CBSE




