
: The glass tube in simple barometer is
(A) Open at both ends
(B) Close at one end
(C) Close at both ends
(D) None
Answer
553.2k+ views
Hint: A barometer is a physical measuring instrument which is used to measure atmospheric pressure. The given question is related to the experimental set up of a simple barometer. We should know the simple barometer experiment set up to answer this question.
Complete step by step solution:
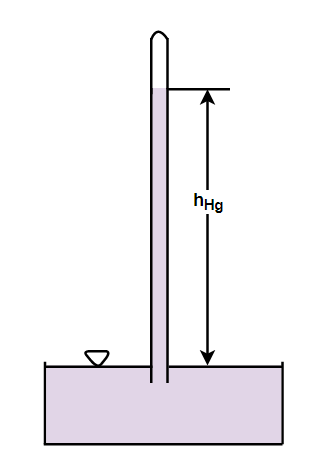
A simple mercury barometer consists of a 1 meter long- clear, dry, thick-walled glass tube. The glass tube is filled with mercury which was sealed at one end. No air bubbles should be present in the mercury column while filling the tube. With your thumb close the open end and carefully turn the tube which is filled with mercury upside down. The mercury inside the tube should not spill out when the tube is in an upside down position, so lock the open end tightly. On the other side take a mercury bath (liquid mercury in a beaker). Dip the open end of the tube which was locked by the thumb, under the mercury bath and remove the thumb.
The mercury level in the tube falls vertically. The level of the mercury in the tube increases or decreases depending on the atmospheric pressure acting on the surface of the mercury. The empty space in the tube above the mercury column is known as the Torricellian vacuum
From the above discussion we can see that the glass tube in the simple barometer is closed at one end and open at the other end.
Hence the correct answer is option (B) close at one end
Note: Mercury is used in simple barometers because it is high density as it is a metal in liquid form. The atmospheric pressure on top of mountains is less than normal atmospheric pressure at sea level. The atmospheric pressure falls down if we move upward.
Complete step by step solution:

A simple mercury barometer consists of a 1 meter long- clear, dry, thick-walled glass tube. The glass tube is filled with mercury which was sealed at one end. No air bubbles should be present in the mercury column while filling the tube. With your thumb close the open end and carefully turn the tube which is filled with mercury upside down. The mercury inside the tube should not spill out when the tube is in an upside down position, so lock the open end tightly. On the other side take a mercury bath (liquid mercury in a beaker). Dip the open end of the tube which was locked by the thumb, under the mercury bath and remove the thumb.
The mercury level in the tube falls vertically. The level of the mercury in the tube increases or decreases depending on the atmospheric pressure acting on the surface of the mercury. The empty space in the tube above the mercury column is known as the Torricellian vacuum
From the above discussion we can see that the glass tube in the simple barometer is closed at one end and open at the other end.
Hence the correct answer is option (B) close at one end
Note: Mercury is used in simple barometers because it is high density as it is a metal in liquid form. The atmospheric pressure on top of mountains is less than normal atmospheric pressure at sea level. The atmospheric pressure falls down if we move upward.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




